What have we learned about TP53-mutated acute myeloid leukemia?
- PMID: 39562552
- PMCID: PMC11576745
- DOI: 10.1038/s41408-024-01186-5
What have we learned about TP53-mutated acute myeloid leukemia?
Abstract
TP53 is a tumor suppressor gene frequently mutated in human cancers and is generally associated with poor outcomes. TP53 mutations are found in approximately 5% to 10% of patients with de novo acute myeloid leukemia (AML), more frequently observed in elderly patients and those with therapy-related AML. Despite recent advances in molecular profiling and the emergence of targeted therapies, TP53-mutated AML remains a challenge to treat. Current treatment strategies, including conventional chemotherapy, hypomethylating agents, and venetoclax-based therapies, have shown limited efficacy in TP53-mutated AML, with low response rates and poor overall survival. Allogeneic hematopoietic stem cell transplantation is a potentially curative option; however, its efficacy in TP53-mutated AML depends on comorbid conditions and disease status at transplantation. Novel therapeutic modalities, including immune-based therapies, did show promise in early-phase studies but did not translate into effective therapies in randomized controlled trials. This review provides a comprehensive overview of TP53 mutations in AML, outcomes based on allelic burden, clinical implications, and therapeutic challenges.
© 2024. The Author(s).
Conflict of interest statement
Figures

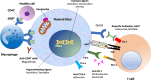
References
-
- Stengel A, Kern W, Haferlach T, Meggendorfer M, Fasan A, Haferlach C. The impact of TP53 mutations and TP53 deletions on survival varies between AML, ALL, MDS and CLL: an analysis of 3307 cases. Leukemia. 2017;31:705–11. - PubMed
-
- Hiwase D, Hahn C, Tran ENH, Chhetri R, Baranwal A, Al-Kali A, et al. TP53 mutation in therapy-related myeloid neoplasm defines a distinct molecular subtype. Blood. 2023;141:1087–91. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

