The resolvin D2 and omega-3 polyunsaturated fatty acid as a new possible therapeutic approach for inflammatory bowel diseases
- PMID: 39562789
- PMCID: PMC11576872
- DOI: 10.1038/s41598-024-80051-8
The resolvin D2 and omega-3 polyunsaturated fatty acid as a new possible therapeutic approach for inflammatory bowel diseases
Abstract
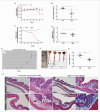
Inflammatory bowel diseases (IBD) are idiopathic disorders characterized by chronic gastrointestinal inflammation. Given conventional therapies' adverse effects and clinical failures, novel approaches are being investigated. Recent studies have highlighted the role of specialized pro-resolving lipid mediators (SPMs) in the active resolution of chronic inflammation. In this regard, omega-3 fatty acid-derived Resolvin D2 (RvD2) appears to play a protective role in the pathophysiology of IBD. Therefore, we characterized the RvD2 pathway and its receptor expression in the intestinal mucosa of experimental colitis induced by dextran sulfate sodium. We also evaluated the preventive impact of an omega-3-enriched diet and the therapeutic efficacy of RvD2 compared with anti-TNF-α treatment. We found an increase in TNFα and IL22 expression and decreased levels of enzymes involved in RvD2 biosynthesis, such as PLA2, 15-LOX, 5-LOX, and its receptor GPR18 in experimental colitis. Omega-3 supplementation reduced the Disease Activity Index (DAI), weight loss, colonic shortening, and inflammation. These results and the increased IL-10 transcriptional levels after RvD2 treatment suggest that this mediator attenuated experimental colitis. These results enhance our understanding of the molecular mechanisms involved in the exacerbated inflammatory response present in experimental colitis and suggest that RvD2 and its omega-3 precursor offer a promising therapeutic approach for IBD.
Keywords: Chronic inflammation; Fatty acid; Inflammatory bowel disease; Omega-3; Pro-resolving lipid mediators; Resolvins.
© 2024. The Author(s).
Conflict of interest statement
Figures



References
MeSH terms
Substances
Grants and funding
- #2332/20 for L.B.P./postdoctoral scholarship from Funding for Education, Research and Extension Support (FAEPEX)
- #2019/07095-3 for B.B.P./Undergraduate scholarship from the FAPESP
- Finance Code 001 for B.L.R./Doctoral scholarship from the Brazilian Coordination for the Improvement of Higher Education Personnel (CAPES Coordenação de Aperfeiçoamento de Pessoal de Nível Superior, Brasil)
- #2022/09393-4 for R.F.L./FAPESP (São Paulo Research Foundation)
- #302557/2021-0 for R.F.L./National Council for Scientific and Technological Development (CNPq)
LinkOut - more resources
Full Text Sources

