Plant-produced SARS-CoV-2 antibody engineered towards enhanced potency and in vivo efficacy
- PMID: 39563066
- PMCID: PMC11672753
- DOI: 10.1111/pbi.14458
Plant-produced SARS-CoV-2 antibody engineered towards enhanced potency and in vivo efficacy
Abstract
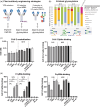
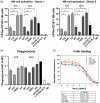
Prevention of severe COVID-19 disease by SARS-CoV-2 in high-risk patients, such as immuno-compromised individuals, can be achieved by administration of antibody prophylaxis, but producing antibodies can be costly. Plant expression platforms allow substantial lower production costs compared to traditional bio-manufacturing platforms depending on mammalian cells in bioreactors. In this study, we describe the expression, production and purification of the originally human COVA2-15 antibody in plants. Our plant-produced mAbs demonstrated comparable neutralizing activity with COVA2-15 produced in mammalian cells. Furthermore, they exhibited similar capacity to prevent SARS-CoV-2 infection in a hamster model. To further enhance these biosimilars, we performed three glyco- and protein engineering techniques. First, to increase antibody half-life, we introduced YTE-mutation in the Fc tail; second, optimization of N-linked glycosylation by the addition of a C-terminal ER-retention motif (HDEL), and finally; production of mAb in plant production lines lacking β-1,2-xylosyltransferase and α-1,3-fucosyltransferase activities (FX-KO). These engineered biosimilars exhibited optimized glycosylation, enhanced phagocytosis and NK cell activation capacity compared to conventional plant-produced S15 and M15 biosimilars, in some cases outperforming mammalian cell produced COVA2-15. These engineered antibodies hold great potential for enhancing in vivo efficacy of mAb treatment against COVID-19 and provide a platform for the development of antibodies against other emerging viruses in a cost-effective manner.
Keywords: SARS‐CoV‐2; antibody; effector function; engineering; glycosylation; structure.
© 2024 The Author(s). Plant Biotechnology Journal published by Society for Experimental Biology and The Association of Applied Biologists and John Wiley & Sons Ltd.
Conflict of interest statement
Véronique Gomord is co‐founder, board member, and CSO of Angany Inc and CEO of Angany InnovationL, Louis‐Philippe Vezina is co‐founder of Angany Inc, Réjean Desgagnés, Loïc Faye, Anne‐Catherine Fitchette, Virginie Catala‐Stordeur, Bertrand Morel, Lucie Mirande, Guillaume Beauverger are employees of Angany Inc. or Angany Innovation.
Figures

References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

