The role of SRP9/SRP14 in regulating Alu RNA
- PMID: 39563162
- PMCID: PMC11581171
- DOI: 10.1080/15476286.2024.2430817
The role of SRP9/SRP14 in regulating Alu RNA
Abstract
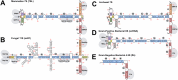
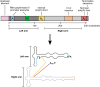
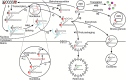
SRP9/SRP14 is a protein heterodimer that plays a critical role in the signal recognition particle through its interaction with the scaffolding signal recognition particle RNA (7SL). SRP9/SRP14 binding to 7SL is mediated through a conserved structural motif that is shared with the primate-specific Alu RNA. Alu RNA are transcription products of Alu elements, a retroelement that comprises ~10% of the human genome. Alu RNA are involved in myriad biological processes and are dysregulated in several human disease states. This review focuses on the roles SRP9/SRP14 has in regulating Alu RNA diversification, maturation, and function. The diverse mechanisms through which SRP9/SRP14 regulates Alu RNA exemplify the breadth of protein-mediated regulation of non-coding RNA.
Keywords: RIG-I; SINE; adenylation; alternative splicing; retrotransposition; scAlu; stress granules; trafficking; translation; viral packaging.
Conflict of interest statement
No potential conflict of interest was reported by the author(s).
Figures
Similar articles
-
Nuclear SRP9/SRP14 heterodimer transcriptionally regulates 7SL and BC200 RNA expression.RNA. 2023 Aug;29(8):1185-1200. doi: 10.1261/rna.079649.123. Epub 2023 May 8. RNA. 2023. PMID: 37156570 Free PMC article.
-
Monomeric scAlu and nascent dimeric Alu RNAs induced by adenovirus are assembled into SRP9/14-containing RNPs in HeLa cells.Nucleic Acids Res. 1996 Nov 1;24(21):4165-70. doi: 10.1093/nar/24.21.4165. Nucleic Acids Res. 1996. PMID: 8932367 Free PMC article.
-
The SRP9/14 subunit of the human signal recognition particle binds to a variety of Alu-like RNAs and with higher affinity than its mouse homolog.Nucleic Acids Res. 1997 Jan 15;25(2):318-26. doi: 10.1093/nar/25.2.318. Nucleic Acids Res. 1997. PMID: 9016560 Free PMC article.
-
Evolutionary history of 7SL RNA-derived SINEs in Supraprimates.Trends Genet. 2007 Apr;23(4):158-61. doi: 10.1016/j.tig.2007.02.002. Epub 2007 Feb 20. Trends Genet. 2007. PMID: 17307271 Review.
-
Alu elements and the human genome.Genetica. 2000;108(1):57-72. doi: 10.1023/a:1004099605261. Genetica. 2000. PMID: 11145422 Review.
Cited by
-
Identification of a minimal Alu domain required for retrotransposition.bioRxiv [Preprint]. 2024 Dec 16:2024.12.16.628748. doi: 10.1101/2024.12.16.628748. bioRxiv. 2024. Update in: Nucleic Acids Res. 2025 Jun 20;53(12):gkaf526. doi: 10.1093/nar/gkaf526. PMID: 39868163 Free PMC article. Updated. Preprint.
-
Identification of a minimal Alu domain required for retrotransposition.Nucleic Acids Res. 2025 Jun 20;53(12):gkaf526. doi: 10.1093/nar/gkaf526. Nucleic Acids Res. 2025. PMID: 40568936 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
