Post-sepsis chronic muscle weakness can be prevented by pharmacological protection of mitochondria
- PMID: 39563237
- PMCID: PMC11577827
- DOI: 10.1186/s10020-024-00982-w
Post-sepsis chronic muscle weakness can be prevented by pharmacological protection of mitochondria
Abstract
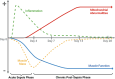
Background: Sepsis, mainly caused by bacterial infections, is the leading cause of in-patient hospitalizations. After discharge, most sepsis survivors suffer from long-term medical complications, particularly chronic skeletal muscle weakness. To investigate this medical condition in detail, we previously developed a murine severe sepsis-survival model that exhibits long-term post-sepsis skeletal muscle weakness. While mitochondrial abnormalities were present in the skeletal muscle of the sepsis surviving mice, the relationship between abnormal mitochondria and muscle weakness remained unclear. Herein, we aimed to investigate whether mitochondrial abnormalities have a causal role in chronic post-sepsis muscle weakness and could thereby serve as a therapeutic target.
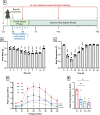
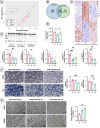
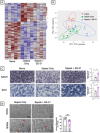
Methods: Experimental polymicrobial abdominal sepsis was induced in 16-18 months old male and female mice using cecal slurry injection with subsequent antibiotic and fluid resuscitation. To evaluate the pathological roles of mitochondrial abnormalities in post-sepsis skeletal muscle weakness, we utilized a transgenic mouse strain overexpressing the mitochondria-specific antioxidant enzyme manganese superoxide dismutase (MnSOD). Following sepsis development in C57BL/6 mice, we evaluated the effect of the mitochondria-targeting synthetic tetrapeptide SS-31 in protecting mitochondria from sepsis-induced damage and preventing skeletal muscle weakness development. In vivo and in vitro techniques were leveraged to assess muscle function at multiple timepoints throughout sepsis development and resolution. Histological and biochemical analyses including bulk mRNA sequencing were used to detect molecular changes in the muscle during and after sepsis RESULTS: Our time course study revealed that post sepsis skeletal muscle weakness develops progressively after the resolution of acute sepsis and in parallel with the accumulation of mitochondrial abnormalities and changes in the mitochondria-related gene expression profile. Transgenic mice overexpressing MnSOD were protected from mitochondrial abnormalities and muscle weakness following sepsis. Further, pharmacological protection of mitochondria utilizing SS-31 during sepsis effectively prevented the later development of muscle weakness.
Conclusions: Our study revealed that the accumulation of mitochondrial abnormalities is the major cause of post-sepsis skeletal muscle weakness. Pharmacological protection of mitochondria during acute sepsis is a potential clinical treatment strategy to prevent post-sepsis muscle weakness.
Keywords: Critical care illness; Mitochondrial myopathy; Muscle weakness; Post-sepsis syndrome.
© 2024. The Author(s).
Conflict of interest statement
Figures


References
-
- Calaf GM. Cancer : oxidative stress and dietary antioxidants. Amsterdam Boston: Elsevier; 2014.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

