Formoterol dynamically alters endocannabinoid tone in the periaqueductal gray inducing headache
- PMID: 39563240
- PMCID: PMC11575070
- DOI: 10.1186/s10194-024-01907-y
Formoterol dynamically alters endocannabinoid tone in the periaqueductal gray inducing headache
Abstract
Background: Headache is a pain disorder present in populations world-wide with a higher incidence in females. Specifically, the incidences of medication overuse headache (MOH) have increased worldwide. Comorbidities of MOH include photosensitivity, anxiety, "brain fog", and decreased physical activity. The FDA-approved long-lasting selective β2-adrenergic receptor agonist, formoterol, is currently approved for use in severe asthma and chronic obstructive pulmonary disease. Recently, interest in repurposing formoterol for use in other disorders including Alzheimer's disease, and neuropathic pain after spinal cord injury and traumatic brain injury has gained traction. Thus, revisiting known side-effects of formoterol, like headache and anxiety, could inform treatment paradigms. The endocannabinoid (eCB) system is implicated in the etiology of preclinical headache, with observed decreases in the circulating levels of endogenous cannabinoids, referred to as Clinical Endocannabinoid Deficiency. As cross-talk between the eCB system and adrenergic receptors has been reported, this study investigated the role of the eCB system and ability of formoterol to induce headache-like periorbital allodynic behavior.
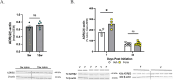
Methods: Female 8-week-old C57Bl/6J mice were treated daily with formoterol (0.3 mg/kg, i.p.) for up to 42-days, during which they were assessed for periorbital allodynia, open field/novel object recognition, and photosensitivity. At the end of the study, the periaqueductal grey (PAG), a brain region known to contribute to both headache induction and maintenance, was collected and subjected to LC-MS to quantify endocannabinoid levels.
Results: Mice exhibited periorbital allodynia at nearly all time points tested and photosensitivity from 28-days onward. Levels of endocannabinoids, anandamide (AEA) and 2-arachidonoylglycerol (2-AG), along with cannabinoid receptor 1 (CB1R) expression were altered by both age and upon treatment with formoterol. Administration of FAAH/MAGL inhibitors, to target the eCB system, and a non-selective cannabinoid receptor agonist, WIN 55,212 reversed the formoterol-induced periorbital allodynia.
Conclusions: These results suggest that formoterol is dysregulates eCB tone to drive headache-like periorbital allodynic behaviors. These results could help inform preventative treatment options for individuals receiving formoterol, as well as provide information on the interaction between the eCB and adrenergic system.
Keywords: Adrenergic; Endocannabinoid; Formoterol; Headache; In vivo; Mouse; PAG.
© 2024. The Author(s).
Conflict of interest statement
Figures





References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

