Development of a core outcome set for psychological therapy trials on acute psychiatric inpatient wards
- PMID: 39563270
- PMCID: PMC11575046
- DOI: 10.1186/s12888-024-06294-x
Development of a core outcome set for psychological therapy trials on acute psychiatric inpatient wards
Abstract
Background: Consensus on what outcomes should be included in trials of psychological therapies on acute psychiatric inpatient wards is currently lacking. Inclusion of different viewpoints, including service user perspectives, is crucial in ensuring that future trials measure outcomes which are meaningful and important. Development of a Core Outcome Set (COS), a minimum standardised set of outcomes to be measured and reported, would help improve synthesis and interpretation of clinical trial data in this area.
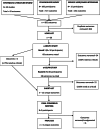
Methods: Stage 1 of the COS development involved compiling a comprehensive long-list of outcomes from key sources including i) a systematic review of outcomes from published trials, ii) online survey of key stakeholders (service users, carers, healthcare professionals, researchers, and end users of research), iii) qualitative interviews with service users and carers. Stage 2 involved stakeholder groups short-listing the outcomes using consensus methods (e-Delphi survey). The final outcome set was derived from the short-list at a consensus meeting of stakeholders, facilitated by an Independent Chair.
Results: A long-list of 68 outcomes was compiled from the systematic review (n = 30 trials), online stakeholder survey (n = 100 participants) and qualitative interviews (n = 15 participants). Fifty stakeholders took part in the e-Delphi study, where the long-list was cut down to a short-list of 12 outcomes over 2 rounds. Nine stakeholders took part in the final consensus meeting, and after some outcomes were removed and/or amalgamated, a final set of 6 outcomes was recommended for inclusion in the COS. These were Ability to Cope, Hopefulness, Quality of Life, Psychosis Symptoms, Mood, and Self-Harm Behaviours.
Conclusions: Widespread future adoption of the COS will reduce research waste by ensuring that outcomes are more easily comparable across trials, and that the full range of stakeholder priorities are represented in trial outcomes. This makes it more likely that effective therapies will be identified in a timely fashion and successfully implemented in routine clinical practice. The final 6-outcome COS should be feasible to implement given the need keep participant burden to a minimum in inpatient trials. Further work is needed to make recommendations for the best outcome measurement instruments to use, including the use of patient-reported outcomes alongside clinician-rated measures.
Trial registration: Not applicable.
Keywords: Clinical Trial; Consensus; Inpatients; Measures.
© 2024. The Author(s).
Conflict of interest statement
Figures
References
-
- Kroenke K, Spitzer RL. The PHQ-9: A new depression diagnostic and severity measure. Psychiatr Ann. 2002;32(9):509–15.
-
- Neil ST, Kilbride M, Pitt L, Nothard S, Welford M, Sellwood W, et al. The questionnaire about the process of recovery (QPR): A measurement tool developed in collaboration with service users. Psychosis. 2009;1(2):145–55.
MeSH terms
LinkOut - more resources
Full Text Sources