Lipoxin A4 improves cardiac remodeling and function in diabetes-associated cardiac dysfunction
- PMID: 39563316
- PMCID: PMC11577589
- DOI: 10.1186/s12933-024-02501-x
Lipoxin A4 improves cardiac remodeling and function in diabetes-associated cardiac dysfunction
Abstract
Background: Diabetic heart disease may eventually lead to heart failure, a leading cause of mortality in diabetic individuals. The lack of effective treatments for diabetes-induced heart failure may result from a failure to address the underlying pathological processes, including chronic, low-grade inflammation. Previous studies have reported that lipoxin A4 (LXA4), known to promote resolution of inflammation, attenuates diabetes-induced atherosclerosis, but its impact on diabetic hearts has not been sought. Thus, we aimed to determine whether LXA4 therapeutic treatment attenuates diabetes-induced cardiac pathology.
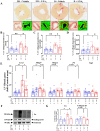
Methods: Six-week-old male apolipoprotein E-deficient (ApoE-/-) mice were followed for 16 weeks after injection of streptozotocin (STZ, 55 mg/kg/day, i.p. for 5 days) to induce type-1 diabetes (T1DM). Treatment with LXA4 (5 μg/kg, i.p.) or vehicle (0.02% ethanol, i.p.) was administered twice weekly for the final 6 weeks. One week before endpoint, echocardiography was performed within a subset of mice from each group. At the end of the study, mice were euthanized with sodium pentobarbital (100 mg/kg i.p.) and hearts were collected for ex vivo analysis, including histological assessment, gene expression profiling by real-time PCR and protein level measurement by western blot.
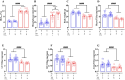
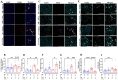
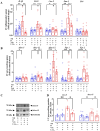
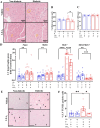
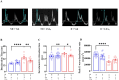
Results: As expected diabetic mice showed a significant elevation in plasma glycated hemoglobin (HbA1c) and glucose levels, along with reduced body weight. Vehicle-treated diabetic mice exhibited increased cardiac inflammation, macrophage content, and an elevated ratio of M1-like to M2-like macrophage markers. In addition, myocardial fibrosis, cardiomyocytes apoptosis and hypertrophy (at the genetic level) were evident, with echocardiography revealing early signs of left ventricular (LV) diastolic dysfunction. Treatment with LXA4 ameliorated diabetes-induced cardiac inflammation, pro-inflammatory macrophage polarization and cardiac remodeling (especially myocardial fibrosis and cardiomyocytes apoptosis), with ultimate improvement in cardiac function. Of note, this improvement was independent of glucose control.
Conclusions: These findings demonstrated that LXA4 treatment attenuated the extent of cardiac inflammation in diabetic hearts, resulting in limited cardiac remodeling and improved LV diastolic function. This supports further exploration of LXA4-based therapy for the management of diabetic heart disease. The recent development of stable LXA4 mimetics holds potential as a novel strategy to treat cardiac dysfunction in diabetes, paving the way for innovative and more effective therapeutic strategies.
Keywords: Cardiac fibrosis; Cardiac inflammation; Diabetic cardiomyopathy; Lipoxin A4; Resolution of inflammation; Specialized pro-resolving lipid mediators.
© 2024. The Author(s).
Conflict of interest statement
Figures
References
-
- Marx N, et al. 2023 ESC guidelines for the management of cardiovascular disease in patients with diabetes. Eur Heart J. 2023;44(39):4043–140. - PubMed
-
- American Diabetes, A., 10. Cardiovascular disease and risk management: standards of medical care in diabetes-2021. Diabetes Care. 2021;44(Suppl 1):S125–S150. - PubMed
-
- Marwick TH, et al. Implications of underlying mechanisms for the recognition and management of diabetic cardiomyopathy. J Am Coll Cardiol. 2018;71(3):339–51. - PubMed
-
- Packer M. Differential pathophysiological mechanisms in heart failure with a reduced or preserved ejection fraction in diabetes. JACC Heart Fail. 2021;9(8):535–49. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

