IL-10 mediates pleural remodeling in systemic lupus erythematosus
- PMID: 39563376
- PMCID: PMC11575414
- DOI: 10.1186/s12964-024-01911-4
IL-10 mediates pleural remodeling in systemic lupus erythematosus
Abstract
Background: Interleukin-10 (IL-10), a pivotal anti-inflammatory cytokine, has gotten attention for its involvement in tissue remodeling and organ fibrosis. Pleurisy and subsequent pleural remodeling are recognized as quantifiable indicators of systemic lupus erythematosus (SLE) activity. However, the role of IL-10 in SLE-associated pleural remodeling remains unknown. In this study, we investigated role of IL-10 in SLE-associated pleural remodeling and the underlying mechanism.
Methods: Clinical data and serum specimens were obtained from SLE patients, while pleural mesothelial cells and mouse models served as primary experimental subjects. The protein expression-related technologies, histopathological staining, and other experimental methods were used in the study.
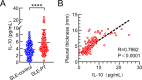
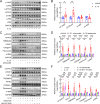
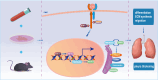
Results: Our investigation got several key findings. Firstly, serum obtained from SLE patients with pleural thickening was found to induce pleural mesothelial cell remodeling. Subsequently, heightened levels of IL-10 were found in serum from SLE patients with pleural thickening compared to that of SLE patients without pleural thickening. Secondly, administration of recombinant IL-10 was confirmed its ability to induce pleural mesothelial cell remodeling, on the contrary, this remodeling was effectively mitigated by IL-10 inhibition. Notably, blockade of IL-10 significantly prevented collagen deposition and prevented thickening in pleura of SLE mouse models. Lastly, the IL-10/JAK2/STAT3/HIF1α/TMEM45A/P4HA1 signaling axis was elucidated to mediate pleural remodeling and thickening.
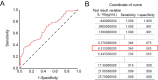
Conclusions: Our study uncovered that IL-10 mediated pleural remodeling in SLE. We suggested that serum IL-10 level exceeding 6.32 pg/mL was a potential reference threshold for predicting pleural thickening in SLE patients.
Keywords: IL-10; Pleural remodeling; Systemic lupus erythematosus (SLE).
© 2024. The Author(s).
Conflict of interest statement
Figures


References
-
- Polachek A, Gladman DD, Su J, Urowitz MB. Defining low Disease activity in systemic Lupus Erythematosus. Arthritis Care Res (Hoboken). 2017;69:997–1003. - PubMed
-
- Torre O, Harari S. Pleural and pulmonary involvement in systemic lupus erythematosus. Presse Med. 2011;40:e19–29. - PubMed
-
- Fanouriakis A, Kostopoulou M, Alunno A, Aringer M, Bajema I, Boletis JN, et al. 2019 update of the EULAR recommendations for the management of systemic lupus erythematosus. Ann Rheum Dis. 2019;78:736–45. - PubMed
-
- Zucchi D, Elefante E, Calabresi E, Signorini V, Bortoluzzi A, Tani C. One year in review 2019: systemic lupus erythematosus. Clin Exp Rheumatol. 2019;37:715–22. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

