Interplay between CO2 and light governs carbon partitioning in Chlamydomonas reinhardtii
- PMID: 39563411
- PMCID: PMC11659805
- DOI: 10.1111/ppl.14630
Interplay between CO2 and light governs carbon partitioning in Chlamydomonas reinhardtii
Abstract
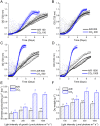
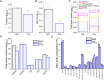
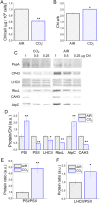
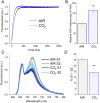
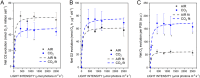
Increasing CO2 availability is a common practice at the industrial level to trigger biomass productivity in microalgae cultures. Still, the consequences of high CO2 availability in microalgal cells exposed to relatively high light require further investigation. Here, the photosynthetic, physiologic, and metabolic responses of the green microalga model Chlamydomonas reinhardtii were investigated in high or low CO2 availability conditions: high CO2 enabled higher biomass yields only if sufficient light energy was provided. Moreover, cells grown in high light and high CO2 availability were characterized, compared to cells grown in high light and low CO2, by a relative increase of the energy-dense triacylglycerols and decreased starch accumulation per dry weight. The photosynthetic machinery adapted to the increased carbon availability, modulating Photosystem II light-harvesting efficiency and increasing Photosystem I photochemical activity, which shifted from being acceptor side to donor side limited: cells grown at high CO2 availability were characterized by increased photosynthetic linear electron flow and by the onset of a balance between NAD(P)H oxidation and NAD(P)+ reduction. Mitochondrial respiration was also influenced by the conditions herein applied, with reduced respiration through the cytochrome pathway compensated by increased respiration through alternative pathways, demonstrating a different use of the cellular reducing power based on carbon availability. The results suggest that at high CO2 availability and high irradiance, the reducing power generated by the oxidative metabolism of photosynthates is either dissipated through alternative oxidative pathways in the mitochondria or translocated back to the chloroplasts to support carbon assimilation and energy-rich lipids accumulation.
© 2024 The Author(s). Physiologia Plantarum published by John Wiley & Sons Ltd on behalf of Scandinavian Plant Physiology Society.
Figures



References
-
- Allorent, G. , Tokutsu, R. , Roach, T. , Peers, G. , Cardol, P. , Girard‐Bascou, J. , Seigneurin‐Berny, D. , Petroutsos, D. , Kuntz, M. , Breyton, C. , Franck, F. , Wollman, F. A. , Niyogi, K. K. , Krieger‐Liszkay, A. , Minagawa, J. & Finazzi, G. 2013. A dual strategy to cope with high light in Chlamydomonas reinhardtii. Plant Cell, 25, 545–57. - PMC - PubMed
-
- Alric, J. 2014. Redox and ATP control of photosynthetic cyclic electron flow in Chlamydomonas reinhardtii: (II) involvement of the PGR5‐PGRL1 pathway under anaerobic conditions. Biochim Biophys Acta, 1837, 825–34. - PubMed
-
- Aronsson, H. , Schöttler, M. A. , Kelly, A. A. , Sundqvist, C. , Dörmann, P. , Karim, S. & Jarvis, P. 2008. Monogalactosyldiacylglycerol deficiency in Arabidopsis affects pigment composition in the prolamellar body and impairs thylakoid membrane energization and photoprotection in leaves. Plant Physiol, 148, 580–92. - PMC - PubMed
-
- Bailleul, B. , Berne, N. , Murik, O. , Petroutsos, D. , Prihoda, J. , Tanaka, A. , Villanova, V. , Bligny, R. , Flori, S. , Falconet, D. , Krieger‐Liszkay, A. , Santabarbara, S. , Rappaport, F. , Joliot, P. , Tirichine, L. , Falkowski, P. , Cardol, P. , Bowler, C. & Finazzi, G. 2015. Energetic coupling between plastids and mitochondria drives CO2 assimilation in diatoms. Nature, 524, 366‐+. - PubMed
-
- Bailleul, B. , Cardol, P. , Breyton, C. & Finazzi, G. 2010. Electrochromism: a useful probe to study algal photosynthesis. Photosynth Res, 106, 179–89. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

