A phase 1 randomized controlled trial of a peptide-based group A streptococcal vaccine in healthy volunteers
- PMID: 39563457
- PMCID: PMC11577953
- DOI: 10.1186/s13063-024-08634-4
A phase 1 randomized controlled trial of a peptide-based group A streptococcal vaccine in healthy volunteers
Abstract
Background: Group A streptococci (Strep A) orStreptococcus pyogenes is a major human pathogen causing an estimated 500,000 deaths worldwide each year. Disease can range from mild pharyngitis to more severe infections, such as necrotizing fasciitis, septicemia, and toxic shock syndrome. Untreated, Strep A infection can lead to the serious post streptococcal pathologies of rheumatic fever/rheumatic heart disease and post-streptococcal glomerulonephritis. An effective vaccine against Strep A would have great benefits worldwide. Here, we test two products, J8 and p*17-both peptide derivatives of a highly conserved region in the M protein, in combination with the protein subunit K4S2 of SpyCEP, an IL-8 protease associated with neutrophil chemoattraction. Each peptide is individually conjugated to cross reacting material (CRM197), and the conjugated peptide vaccines are abbreviated as J8-K4S2 or p*17-K4S2.
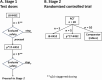
Methods: This single-site phase I, two-stage clinical trial in Edmonton, Alberta, Canada, aims to recruit a total of 30 healthy volunteers, aged 18-45 years, without any evidence of pre-existing valvular heart disease. The trial is divided into the initial unblinded safety test dose stage (stage 1) and the randomized, double-blinded, controlled trial stage (stage 2). Stage 1 will recruit 10 volunteers-5 each to receive either J8-K4S2 or p*17-K4S2 in an unblinded, staggered fashion, whereby volunteers are dosed with intentional spacing of at least 2 days in between doses to monitor for any immediate side effects before dosing the next. Once all 5 volunteers have received 3 doses of the first test vaccine, a similar process will follow for the second test vaccine. Once safety is established in stage 1, we will proceed to stage 2, which will recruit 20 volunteers to our 3-arm randomized controlled trial (RCT), receiving either of the trial vaccines, J8-K4S2 or p*17-K4S2, or comparator (rabies) vaccine. All product dosing will be at 0, 3, and 6 weeks. The primary outcome is vaccine safety; the secondary outcome is immunogenicity and comparative analyses of the different vaccine regimens.
Discussion: This Strep A vaccine clinical trial aims to investigate safety and immunogenicity of two novel conjugated peptide-based vaccines, J8-KS42 and p*17-K4S2. If one or both vaccine products demonstrate favorable primary and secondary outcomes, the product(s) will move into phase II and III studies.
Trial registration: ClinicalTrials.gov Identifier: NCT04882514. Registered on 2021-05-12, https://clinicaltrials.gov/study/NCT04882514 .
Keywords: Streptococcus pyogenes; Group A streptococcus (GAS; Strep a); M protein; Phase I vaccine clinical trial; Spy-CEP.
© 2024. The Author(s).
Conflict of interest statement
Figures
References
-
- WHO. World Health Organization (15 December 2022). Disease outbreak news; increased incidence of scarlet fever and invasive group A Streptococcus infection - multi-country. 2022. https://www.who.int/emergencies/disease-outbreak-news/item/2022-DON429.
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical