Porous pillar[6]arene-based polymers for reversible iodine capture
- PMID: 39563709
- PMCID: PMC11575550
- DOI: 10.1039/d4na00667d
Porous pillar[6]arene-based polymers for reversible iodine capture
Abstract
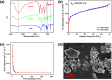
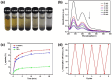
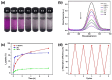
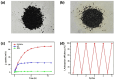
Iodine in nuclear waste can cause serious environment pollution and health risks, and has thus driven more development of materials for iodine capture. Herein, a novel porous pillar[6]arene-based polymer (P-P6APs) was easily prepared as a supramolecular adsorbent for iodine via a one-step crosslinking reaction between per-hydroxylated pillar[6]arene and decafluorobiphenyl. Nitrogen adsorption tests demonstrated that this material possessed a satisfactory surface area (S BET = 366 m2 g-1) and pore diameter (3.8 nm), which was due to the macrocyclic scaffolds. Compared with commercially available activated carbon, P-P6APs exhibited superior adsorption efficiency toward volatile iodine not only in the solution phase (water and n-hexane) but also in the gas phase. This outcome was mainly ascribed to nonspecific adsorption by the pore structure of the crosslinked material coupled with multiple intermolecular binding sites of the macrocyclic scaffold. Moreover, this supramolecular absorbent was recyclable and could be reused 5 times with no obvious loss of performance.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures

Similar articles
-
Imidazole Cationic-Bridged Pillar[5]arene Polymer as a Recycle Adsorbent for Iodine Capture.ACS Appl Mater Interfaces. 2025 Feb 5;17(5):8382-8393. doi: 10.1021/acsami.4c21105. Epub 2025 Jan 23. ACS Appl Mater Interfaces. 2025. PMID: 39849298
-
Reversible Iodine Capture by Nonporous Pillar[6]arene Crystals.J Am Chem Soc. 2017 Nov 1;139(43):15320-15323. doi: 10.1021/jacs.7b09850. Epub 2017 Oct 24. J Am Chem Soc. 2017. PMID: 29035524
-
Applications of Supramolecular Polymers Generated from Pillar[n]arene-Based Molecules.Polymers (Basel). 2023 Nov 27;15(23):4543. doi: 10.3390/polym15234543. Polymers (Basel). 2023. PMID: 38231964 Free PMC article. Review.
-
Pillar[5,6]arene-functionalized silicon dioxide: synthesis, characterization, and adsorption of herbicide.Langmuir. 2015 Feb 3;31(4):1454-61. doi: 10.1021/la5050199. Epub 2015 Jan 16. Langmuir. 2015. PMID: 25557460
-
Pillar[n]arene-Mimicking/Assisted/Participated Carbon Nanotube Materials.Materials (Basel). 2022 Sep 3;15(17):6119. doi: 10.3390/ma15176119. Materials (Basel). 2022. PMID: 36079500 Free PMC article. Review.
References
-
- Zhan L. Bo Y. Lin T. Fan Z. Development and outlook of advanced nuclear energy technology. Energy Strategy Rev. 2021;34:100630. doi: 10.1016/j.esr.2021.100630. - DOI
-
- Adamantiades A. Kessides I. Nuclear power for sustainable development: Current status and future prospects. Energy Policy. 2009;37:5149–5166. doi: 10.1016/j.enpol.2009.07.052. - DOI
-
- Sadiq M. Shinwari R. Usman M. Ozturk I. Maghyereh A. I. Linking nuclear energy, human development and carbon emission in BRICS region: Do external debt and financial globalization protect the environment? Nucl. Eng. Technol. 2022;54:3299–3309. doi: 10.1016/j.net.2022.03.024. - DOI
-
- Wu Y. Xie Y. Liu X. Li Y. Wang J. Chen Z. Yang H. Hu B. Shen C. Tang Z. Huang Q. Wang X. Functional nanomaterials for selective uranium recovery from seawater: Material design, extraction properties and mechanisms. Coord. Chem. Rev. 2023;483:215097. doi: 10.1016/j.ccr.2023.215097. - DOI
LinkOut - more resources
Full Text Sources

