Evaluating the impact of redox potential on the growth capacity of anaerobic gut fungi
- PMID: 39563712
- PMCID: PMC11575491
- DOI: 10.1093/femsmc/xtae033
Evaluating the impact of redox potential on the growth capacity of anaerobic gut fungi
Abstract
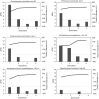
Anaerobic gut fungi (AGF, Neocallimastigomycota) inhabit the alimentary tract of herbivores. Although strict anaerobes, studies have suggested their capacity to retain viability after various durations of air exposure. It is currently unclear whether AGF can actively grow, and not merely survive, in redox potentials (Eh) higher than those encountered in the herbivorous gut. We evaluated the growth of two AGF strains (Orpinomyces joyonii and Testudinimyces gracilis) at various Eh levels, achieved by manipulating the concentrations of reductant (cysteine hydrochloride) in culture media. Both strains exhibited robust and sustainable growth at negative Eh (-50 mV or below). However, growth in the absence of cysteine hydrochloride (Eh value around +50 mV) was possible only for O. joyonii and only for one subcultivation. The capacity to grow at +50 mV was further confirmed in four additional taxa (Pecoramyces ruminatium, Anaeromyces mucronatus, Aklioshbmyces papillarum, and Piromyces communis), while two (Aestipascuomyces dupliciliberans and Capellomyces foraminis) failed to grow under these conditions. Our results establish the ability of AGF to grow at redox potential values higher than those encountered in their natural habitats. Such capability could contribute to efficient AGF dispersal and horizontal transmission between hosts, and could have important implications for industrial applications of AGF.
Keywords: anaerobic fungi; anaerobiosis; redox potential.
© The Author(s) 2024. Published by Oxford University Press on behalf of FEMS.
Conflict of interest statement
None declared.
Figures



References
-
- Akhmanova A, Voncken F, Hosea Ket al. . A hydrogenosome with pyruvate formate-lyase: anaerobic chytrid fungi use an alternative route for pyruvate catabolism. Mol Microbiol. 1999;32:1103–14. - PubMed
-
- Boxma B, Voncken F, Jannink Set al. . The anaerobic chytridiomycete fungus Piromyces sp. E2 produces ethanol via pyruvate:formate lyase and an alcohol dehydrogenase E. Mol Microbiol. 2004;51:1389–99. - PubMed
-
- Brookman J, Ozkose E, Rogers Set al. . Identification of spores in the polycentric anaerobic gut fungi which enhance their ability to survive. FEMS Microbiol Ecol. 2000;31:261–7. - PubMed
-
- Calkins S, Elledge N, Hanafy Ret al. . A fast and reliable procedure for spore collection from anaerobic fungi: Application for RNA uptake and long-term storage of isolates. J Microbiol Methods. 2016;127:206–13. - PubMed
LinkOut - more resources
Full Text Sources
