Thanatin and vinyl sulfide analogues as narrow spectrum antimicrobial peptides that synergise with polymyxin B
- PMID: 39564120
- PMCID: PMC11573584
- DOI: 10.3389/fphar.2024.1487338
Thanatin and vinyl sulfide analogues as narrow spectrum antimicrobial peptides that synergise with polymyxin B
Abstract
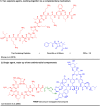
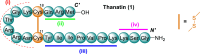
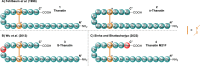
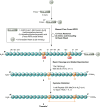
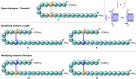
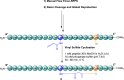
Thanatin is a β-hairpin antimicrobial peptide cyclised by a single disulfide bond that has shown potent broad-spectrum activity towards bacterial and fungal pathogens. Towards Gram-negative species, thanatin acts both by forming trans-membranal pores and inhibiting outer membrane biogenesis by binding to LptA and blocking lipopolysaccharide (LPS) transport. Inspired by previous modifications of thanatin, an analogue was prepared which demonstrated potent but selective activity towards E. coli. Furthermore, this compound was shown to act in synergy with the highly potent FDA-approved lipopeptide antibiotic polymyxin B, which engages LPS at the cytoplasmic membrane. Four analogues of thanatin in which the disulfide was substituted for vinyl sulfide bridge mimetics were prepared, all of which retained similar secondary structures. Two of these retained substantial potency and selectivity towards E. coli. Importantly, synergy with polymyxin B was also maintained for the lead analogue. The vinyl sulfide potentially offers a facile replacement strategy for labile disulfide bonds and the selective activity and drug synergy of the reported thanatin analogues is promising for the development of narrow spectrum antimicrobials with reduced likelihood of resistance emerging in clinical settings.
Keywords: AMP; antibiotic synergy; polymyxin B; thanatin; vinyl sulfide; β-hairpin.
Copyright © 2024 Shepperson, Harris, Brimble and Cameron.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
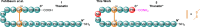

Similar articles
-
Single Disulfide Bond in Host Defense Thanatin Analog Peptides: Antimicrobial Activity, Atomic-Resolution Structures and Target Interactions.Int J Mol Sci. 2024 Dec 24;26(1):51. doi: 10.3390/ijms26010051. Int J Mol Sci. 2024. PMID: 39795909 Free PMC article.
-
Outer-Membrane Permeabilization, LPS Transport Inhibition: Activity, Interactions, and Structures of Thanatin Derived Antimicrobial Peptides.Int J Mol Sci. 2024 Feb 9;25(4):2122. doi: 10.3390/ijms25042122. Int J Mol Sci. 2024. PMID: 38396798 Free PMC article.
-
Linking dual mode of action of host defense antimicrobial peptide thanatin: Structures, lipopolysaccharide and LptAm binding of designed analogs.Biochim Biophys Acta Biomembr. 2022 Mar 1;1864(3):183839. doi: 10.1016/j.bbamem.2021.183839. Epub 2021 Dec 13. Biochim Biophys Acta Biomembr. 2022. PMID: 34915021
-
Thanatin: An Emerging Host Defense Antimicrobial Peptide with Multiple Modes of Action.Int J Mol Sci. 2021 Feb 3;22(4):1522. doi: 10.3390/ijms22041522. Int J Mol Sci. 2021. PMID: 33546369 Free PMC article. Review.
-
Thanatin: A Promising Antimicrobial Peptide Targeting the Achilles' Heel of Multidrug-Resistant Bacteria.Int J Mol Sci. 2024 Aug 31;25(17):9496. doi: 10.3390/ijms25179496. Int J Mol Sci. 2024. PMID: 39273441 Free PMC article. Review.
Cited by
-
Broad-Spectrum Gramicidin S Derivatives with Potent Activity Against Multidrug-Resistant Gram-Negative ESKAPE Pathogens.Antibiotics (Basel). 2025 Apr 22;14(5):423. doi: 10.3390/antibiotics14050423. Antibiotics (Basel). 2025. PMID: 40426491 Free PMC article.
-
Single Disulfide Bond in Host Defense Thanatin Analog Peptides: Antimicrobial Activity, Atomic-Resolution Structures and Target Interactions.Int J Mol Sci. 2024 Dec 24;26(1):51. doi: 10.3390/ijms26010051. Int J Mol Sci. 2024. PMID: 39795909 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

