Perfluorohexyloctane ophthalmic solution for dry eye disease: pooled analysis of two phase 3 clinical trials
- PMID: 39564145
- PMCID: PMC11575077
- DOI: 10.3389/fopht.2024.1452422
Perfluorohexyloctane ophthalmic solution for dry eye disease: pooled analysis of two phase 3 clinical trials
Abstract
Background: Dry eye disease (DED) is commonly caused by excessive tear film evaporation due to Meibomian gland dysfunction (MGD). There is a need for DED treatment options that address tear evaporation and benefit patients across a broad range of demographic and disease characteristics. This study evaluated treatment effects of perfluorohexyloctane ophthalmic drop (formerly NOV03) in the pooled dataset from 2 pivotal clinical trials in patients with DED associated with MGD, both in the overall population and in patient subgroups based on sex, age, and baseline severity of eye dryness.
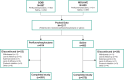
Methods: Pooled data from 2 similarly designed, phase 3, randomized controlled trials (GOBI, MOJAVE) were analyzed. Patients aged ≥18 years with DED administered perfluorohexyloctane (n=614) or hypotonic (0.6% solution) saline control (n=603) four times daily for 8 weeks. Primary endpoints were total corneal fluorescein staining (tCFS) score (National Eye Institute scale, 0-15) and eye dryness visual analog scale (VAS) score (0-100). Efficacy was evaluated using analysis of covariance among patient subgroups (male and female, older [≥65 years] and younger [18 to <65 years], tCFS score <7 and ≥7, VAS eye dryness score <70 and ≥70, MGD score <7 and ≥7, Schirmer I test <10 mm and ≥10 mm).
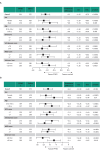
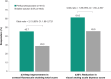
Results: Reductions in tCFS and VAS eye dryness scores were greater for perfluorohexyloctane versus control. In the overall patient population, least-squares mean treatment difference was -1.1 (95% CI: -1.41 to -0.79; p<0.0001) for tCFS and -9.0 (95% CI: -11.90 to -6.00; p<0.0001) for VAS eye dryness. Treatment favored perfluorohexyloctane over control in all patient subgroup analyses of tCFS and VAS eye dryness. Overall, the most common adverse event with perfluorohexyloctane was blurred vision (2.1% of patients), which was mild and transient.
Conclusions: Compared with a hypotonic saline control, perfluorohexyloctane improved both the signs and symptoms of DED, including in patients with greater self-reported severity of eye dryness.
Clinical trial registration: This study represents an integrated analysis of 2 previous clinical trials: GOBI (ClinicalTrials.gov, NCT04139798) and MOJAVE (ClinicalTrials.gov, NCT04567329).
Keywords: Meibomian gland dysfunction; NOV03; clinical trial; dry eye disease; perfluorohexyloctane; tear film evaporation.
Copyright © 2024 Fahmy, Harthan, Evans, Greiner, Tauber, Sheppard, Krösser and Vittitow.
Conflict of interest statement
AF was employed by company Minnesota Eye Consultants. DE was employed by company Total Eye Care. JT was employed by company Tauber Eye Center. JS was employed by company Virginia Eye Consultants. AF reports serving on advisory boards, speakers bureaus, or as a consultant for Allergan, BioTissue, Dompé, Lumenis Be, NovaBay, Oyster Point Pharma, and Sight Sciences. JH reports serving on advisory boards or as an investigator for Bausch + Lomb, Novartis, Johnson & Johnson, Kala Pharmaceuticals, and Ocular Therapeutix. DE reports serving on advisory board for Visus Therapeutics and reports presently serving as an investigator for Alcon Research, Bausch + Lomb, Glaukos, Johnson & Johnson Vision, Kowa, Mimetogen Pharmaceuticals, Novartis, Ocular Therapeutix, Oculis, Stuart Therapeutics, and Visus Therapeutics. JG reports presently serving as an investigator for Allergan, Bausch + Lomb, Calm Water Therapeutics, LENZ Therapeutics, Mimetogen Pharmaceuticals, and Sylentis. JT reports serving as an investigator for Alcon, Aramis Biosciences, Bausch + Lomb, Claris Bio, Novaliq, Novartis, Oyster Point Pharma, Sylentis, Tarsus, TearSolutions, Trefoil Therapeutics, and Visus Therapeutics. JS reports serving on advisory boards, speakers bureaus, or as a consultant for 1-800-DOCTORS, AbbVie, Aerie, Alcon, Aldeyra Therapeutics, Allergan, ArcScan, Avedro, Bausch + Lomb, Bio-Tissue/TissueTech, BioLayer, Bruder Healthcare, Claris Bio, Clearside Biomedical, Clearview, Clementia Pharmaceuticals, Dompé, Eleven Optical, Eyedetec Medical, EyeGate, Eyevance Pharmaceuticals, Fortress Biotech, Glaukos, Hovione, Imprimis Pharmaceuticals, Inspire Pharmaceuticals/Merck & Co, Inc, Isis (Ionis) Pharmaceuticals, Johnson & Johnson/TearScience/Vistakon, Kala Pharmaceuticals, Kowa Pharmaceuticals America, LacriSciences Vision, LayerBio, Lenstatin, Lumenis Be, Lux Biosciences, Mallinckrodt, Mati Therapeutics, MedEdicus, Mitotech, SA, Nicox, NovaBay Pharmaceuticals, Novaliq GmbH, Novartis Pharmaceuticals, Noveome Biotherapeutics/Stemnion, OcuCure Therapeutics, Ocular Therapeutix, Oculis, Okogen, Omeros, Oyster Point Pharma, Parion Sciences, PentaVision, Pfizer, Portage, Quidel, Rapid Pathogen Screening, Santen Pharmaceutical, ScienceBased Health, Shire, Sun Pharmaceutical Industries, Surrozen, Synergel, Takeda Pharmaceutical, Talia Technology, TearLab, TearSolutions, Topcon Healthcare, and TopiVert Pharma; conducting clinical research for AbbVie, Alcon, Aldeyra Therapeutics, Allergan, ArcScan, Avedro, Bausch + Lomb, Clearside Biomedical, Clearview, Clementia Pharmaceuticals, Dompé, EyeGate Research, EyeRx Research, Fortress Biotech, Glaukos, Hovione, InSite Vision, Inspire Pharmaceuticals/Merck & Co, Inc, Ionis Pharmaceuticals, Johnson & Johnson/TearScience/Vistakon, Kala Pharmaceuticals, LacriSciences Vision, Lux Biosciences, Mimetogen Pharmaceuticals, Mitotech, SA, NeoMedix Corporation, Novaliq GmbH, Novartis Pharmaceuticals, Ocular Therapeutix, Okogen, Oyster Point Pharma, Parion Sciences, Pfizer, Rapid Pathogen Screening, Rutech, Santen Pharmaceutical, Senju Pharmaceutical, Shire, TearSolutions, Topcon Healthcare, and XOMA/Servier; investor, shareholder, or stock interests in 1-800-DOCTORS, Alphaeon (parent company: Strathspey Crown), BioLayer, Eyedetec Medical, EyeGate Research, EyeRx Research, Eyevance Pharmaceuticals, LacriSciences Vision, LayerBio, Mati Therapeutics, NovaBay Pharmaceuticals, Noveome Biotherapeutics/Stemnion, OccuHub, OcuCure Therapeutics, Okogen, Oyster Point Pharma, Rapid Pathogen Screening, Shire, and TearLab. SK is an employee of Novaliq GmbH. JV is an employee of Bausch + Lomb.
Figures

References
Associated data
LinkOut - more resources
Full Text Sources
Medical

