Cytochrome c and cancer cell metabolism: A new perspective
- PMID: 39564377
- PMCID: PMC11570848
- DOI: 10.1016/j.jsps.2024.102194
Cytochrome c and cancer cell metabolism: A new perspective
Abstract
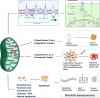
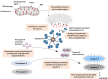
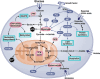
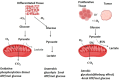
Cytochrome c is a vital electron carrier in the mitochondrial respiratory chain. When the outer membrane of mitochondria becomes permeable, cytochrome c is discharged into the cytoplasm, where it initiates the intrinsic apoptosis pathway. The complex interaction between cytochrome c and apoptosis protease-activating factor-1 (Apaf-1) leads to the formation of the apoptosome and activation of a cascade of caspases, highlighting the critical role of cytochrome c in controlling cell death mechanisms. Additionally, cytochrome c undergoes post-translational modifications, especially phosphorylation, which intricately regulate its roles in both respiration and apoptosis. These modifications add layers of complexity to how cytochrome c effectively controls cellular functions. cytochrome c becomes a lighthouse in the intricate web of cancer, its expression patterns providing hints about prognosis and paths toward treatment. Reduced levels of cytochrome c have been observed in cancer tissues, indicating a potential inhibition of apoptosis. For instance, in glioma tissues, cytochrome c levels were lower compared to healthy tissues, and this reduction became more pronounced in advanced stages of the disease. However, the role of cytochrome c in cancer becomes more intricate as it becomes intertwined with the metabolic reprogramming of cancer cells. This suggests that cytochrome c plays a crucial role in tumor progression and resistance to treatment. Viewing cytochrome c as a molecular mosaic reveals that it is not merely a protein, but also a central player in determining cellular fate. This realization opens up exciting avenues for potential advancements in cancer diagnosis and treatment strategies. Despite the advancements made, the narrative surrounding cytochrome c remains incomplete, urging further exploration into its complexities and the biological implications linked to cancer. cytochrome c stands as a beacon of hope and a promising target for therapy in the battle against cancer, particularly due to its significant involvement in tumor metabolism. It holds the potential for a future where innovative solutions can be developed to address the intricate challenges of cellular fate. In this review, we have endeavored to illuminate the multifaceted domain of cytochrome c drawing connections among apoptosis, metabolic reprogramming, and the Warburg effect in the context of cancer.
Keywords: And cancer; Apoptosis; Apoptosome; Cancer; Cytochrome c; Oxidative phosphorylation; Warburg effect.
© 2024 The Author(s).
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures


Similar articles
-
Transforming growth factor-beta(1) induces apoptosis in rat FaO hepatoma cells via cytochrome c release and oligomerization of Apaf-1 to form a approximately 700-kd apoptosome caspase-processing complex.Hepatology. 2000 Oct;32(4 Pt 1):750-60. doi: 10.1053/jhep.2000.18329. Hepatology. 2000. PMID: 11003619
-
Chemical-induced apoptosis: formation of the Apaf-1 apoptosome.Drug Metab Rev. 2003 Nov;35(4):337-63. doi: 10.1081/dmr-120026497. Drug Metab Rev. 2003. PMID: 14705865 Review.
-
Pro-apoptotic proteins released from the mitochondria regulate the protein composition and caspase-processing activity of the native Apaf-1/caspase-9 apoptosome complex.J Biol Chem. 2004 May 7;279(19):19665-82. doi: 10.1074/jbc.M311388200. Epub 2004 Mar 1. J Biol Chem. 2004. PMID: 14993223
-
Modeling of interaction between cytochrome c and the WD domains of Apaf-1: bifurcated salt bridges underlying apoptosome assembly.Biol Direct. 2015 May 27;10:29. doi: 10.1186/s13062-015-0059-4. Biol Direct. 2015. PMID: 26014357 Free PMC article.
-
Copper metabolism in cell death and autophagy.Autophagy. 2023 Aug;19(8):2175-2195. doi: 10.1080/15548627.2023.2200554. Epub 2023 Apr 16. Autophagy. 2023. PMID: 37055935 Free PMC article. Review.
Cited by
-
Integrating cuproptosis and immunosenescence: A novel therapeutic strategy in cancer treatment.Biochem Biophys Rep. 2025 Mar 27;42:101983. doi: 10.1016/j.bbrep.2025.101983. eCollection 2025 Jun. Biochem Biophys Rep. 2025. PMID: 40224540 Free PMC article. Review.
References
-
- Anguita E., Villalobo A. Ca2+ signaling and Src-kinases-controlled cellular functions. Arch. Biochem. Biophys. 2018;650:59–74. - PubMed
-
- Barczyk K., et al. Serum cytochrome c indicates in vivo apoptosis and can serve as a prognostic marker during cancer therapy. Int. J. Cancer. 2005;116(2):167–173. - PubMed
-
- Bayir H., et al. Apoptotic interactions of cytochrome c: redox flirting with anionic phospholipids within and outside of mitochondria. Biochimica et Biophysica Acta (BBA)-Bioenergetics. 2006;1757(5–6):648–659. - PubMed
Publication types
LinkOut - more resources
Full Text Sources

