Measles surveillance data analysis and serological survey in Quzhou, China, 2014-2024: an assessment of progress toward measles elimination
- PMID: 39564511
- PMCID: PMC11573565
- DOI: 10.3389/fmed.2024.1492873
Measles surveillance data analysis and serological survey in Quzhou, China, 2014-2024: an assessment of progress toward measles elimination
Abstract
Background: Measles is a disease that can be eliminated through vaccination. In recent years, measles incidence and mortality have been greatly reduced.
Methods: Analyze measles surveillance data from 2014 to 2023 and measles seroepidemiological characteristics of healthy populations in 2024 to assess progress toward measles elimination.
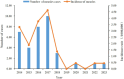
Results: A total of 35 measles cases were reported in the surveillance system from 2014-2023 in Quzhou, with an average annual incidence of 1.6/1 million. Since 2019, the incidence of measles has been lower than 0.5/1 million. A serological survey of 257 healthy people showed that the positive rate of measles IgG antibody was 90.3%, and the immunity of all age groups except 0-5 years old was lower than 95%, which did not reach the threshold of 95% herd immunity required for eliminating measles.
Conclusion: Although the incidence of measles in Quzhou is low, the immunity of healthy people to measles infection is insufficient. Measles is still in the control phase, not in the elimination phase. Inadequate immunity in the population may be due to the failure to achieve ≥95% vaccination coverage and low immunogenicity of the vaccine. Recommends that the quality of routine immunization data be assessed and monitored to verify reported vaccination coverage; at the same time, improve vaccination services and optimize vaccination policies to increase actual vaccination coverage. In addition, it is recommended to adjust the MMR immunization strategy, changing the time of the first MMR vaccination from 8 months of age to 12-15 months of age, and the second dose at 4 to 6 years of age.
Keywords: antibody; elimination; incidence; measles; surveillance; vaccination.
Copyright © 2024 Gong, Zheng, Lai and Yin.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
-
- Wanlapakorn N, Wasitthankasem R, Vichaiwattana P, Auphimai C, Yoocharoen P, Vongpunsawad S, et al. Antibodies against measles and rubella virus among different age groups in Thailand: a population-based serological survey. PLoS One. (2019) 14:e0225606. doi: 10.1371/journal.pone.0225606, PMID: - DOI - PMC - PubMed
-
- Saffar H, Mousavi SJ, Saffar H, Parsaei MR, Ghorbani GR, Saffar MJ. Seroconversion rates following 2 doses of measles-mumps- rubella vaccination given at the ages 12 and 18 months: data for possible additional dose at older age. BMC Immunol. (2022) 23:2. doi: 10.1186/s12865-021-00465-1 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources