A single-chain variable fragment-based bispecific T-cell activating antibody against CD117 enables T-cell mediated lysis of acute myeloid leukemia and hematopoietic stem and progenitor cells
- PMID: 39564539
- PMCID: PMC11574467
- DOI: 10.1002/hem3.70055
A single-chain variable fragment-based bispecific T-cell activating antibody against CD117 enables T-cell mediated lysis of acute myeloid leukemia and hematopoietic stem and progenitor cells
Abstract
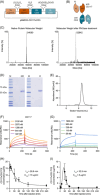
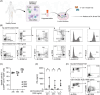
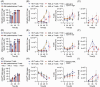
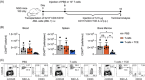
Acute myeloid leukemia (AML) derives from hematopoietic stem and progenitor cells (HSPCs). To date, no AML-exclusive, non-HSPC-expressed cell-surface target molecules for AML selective immunotherapy have been identified. Therefore, to still apply surface-directed immunotherapy in this disease setting, time-limited combined immune-targeting of AML cells and healthy HSPCs, followed by hematopoietic stem cell transplantation (HSCT), might be a viable therapeutic approach. To explore this, we generated a recombinant single-chain variable fragment-based bispecific T-cell engaging and activating antibody directed against CD3 on T-cells and CD117, the surface receptor for stem cell factor, expressed by both AML cells and healthy HSPCs. Bispecific CD117xCD3 targeting induced lysis of CD117-positive healthy human HSPCs, AML cell lines and patient-derived AML blasts in the presence of T-cells at subnanomolar concentrations in vitro. Furthermore, in immunocompromised mice, engrafted with human CD117-expressing leukemia cells and human T-cells, the bispecific molecule efficiently prevented leukemia growth in vivo. Additionally, in immunodeficient mice transplanted with healthy human HSPCs, the molecule decreased the number of CD117-positive cells in vivo. Therefore, bispecific CD117xCD3 targeting might be developed clinically in order to reduce CD117-expressing leukemia cells and HSPCs prior to HSCT.
© 2024 The Author(s). HemaSphere published by John Wiley & Sons Ltd on behalf of European Hematology Association.
Conflict of interest statement
Jonathan D. Kiefer, Renier Myburgh, Dario Neri, and Markus G. Manz are inventors in patent applications that describe the anti‐CD117xCD3 TCE. The commercialization rights/plans for this patent are overseen and regulated by the Technology Transfer Office of the University of Zurich and ETH Zürich, which granted an exclusive license to ATLyphe (www.atlyphe.com). Renier Myburgh, Jonathan D. Kiefer, Norman F. Russkamp, Danielle Villars, Dario Neri/Philogen, and Markus G. Manz are shareholders of ATLyphe. All other authors declare no conflict of interest.
Figures






References
-
- Bonnet D, Dick JE. Human acute myeloid leukemia is organized as a hierarchy that originates from a primitive hematopoietic cell. Nat Med. 1997;3:730‐737. - PubMed
-
- Trumpp A, Haas S. Cancer stem cells: the adventurous journey from hematopoietic to leukemic stem cells. Cell. 2022;185:1266‐1270. - PubMed
-
- Xue S‐A, Gao L, Hart D, et al. Elimination of human leukemia cells in NOD/SCID mice by WT1‐TCR gene–transduced human T cells. Blood. 2005;106:3062‐3067. - PubMed
LinkOut - more resources
Full Text Sources
