Neuromodulation Improves Stress Urinary Incontinence-Like Deficits in Female Rabbits
- PMID: 39564558
- PMCID: PMC11573403
- DOI: 10.1109/OJEMB.2024.3408454
Neuromodulation Improves Stress Urinary Incontinence-Like Deficits in Female Rabbits
Abstract
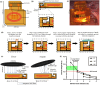
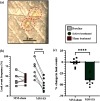
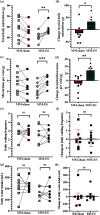
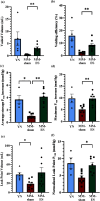
Objective: Stress urinary incontinence (SUI) affects a third of the female population and is characterized by involuntary urine leakage during abdominal efforts such as sneezing, laughing, or coughing. Acute neuromodulation of the bulbospongiosus nerve (BsN) was shown to increase bladder efficiency in aged and multiparous rabbits. This study investigates the efficacy of sub-chronic BsN neuromodulation in alleviating SUI-like deficits in mature multiparous rabbits, characterized by increased urine leakage and reduced leak point pressure. Results: Using the voiding spot assay, we observed a 40% reduction in urine leakage events after 30 days of BsN stimulation, which correlated with a 60% increase in daily micturition volume, a 10-fold increase in voided volume, and improvements in voiding efficiency and leak point pressure compared to negative control animals. Conclusion: In multiparous rabbits, BsN neuromodulation improves important SUI-like metrics including bladder capacity and urethral closure, supporting the use of this bioelectronic modality as treatment for SUI.
Keywords: Bioelectronics; electrical stimulation; neural interfaces; pelvic floor disorders; pelvic innervation.
© 2024 The Authors.
Conflict of interest statement
MR-O owns shares in Juniper Biomedical, a medical device company. FSR is currently an intern at Juniper Biomedical but was not affiliated with the company during the study and data analysis period. Juniper Biomedical did not have any role in animal data collection, analysis, or in the manuscript.
Figures

Similar articles
-
Targeted neuromodulation of pelvic floor nerves in aging and multiparous rabbits improves continence.Sci Rep. 2021 May 19;11(1):10615. doi: 10.1038/s41598-021-90088-8. Sci Rep. 2021. PMID: 34011938 Free PMC article.
-
Secondary urethral sphincter function of the rabbit pelvic and perineal muscles.Front Neurosci. 2023 Feb 16;17:1111884. doi: 10.3389/fnins.2023.1111884. eCollection 2023. Front Neurosci. 2023. PMID: 36875671 Free PMC article.
-
Immediate effects of Zhongji point acupuncture on pelvic floor structure in female patients with stress urinary incontinence: a randomized, single-blind, and sham-controlled clinical trial protocol.Ann Palliat Med. 2021 Jul;10(7):8292-8299. doi: 10.21037/apm-21-662. Epub 2021 Jul 2. Ann Palliat Med. 2021. PMID: 34263647
-
[Bladder injury during sling operation in the treatment of SUI--review of literature and case report].Ginekol Pol. 2012 Oct;83(10):784-8. Ginekol Pol. 2012. PMID: 23383566 Review. Polish.
-
Stress urinary incontinence in women: review and update on neurological control.J Womens Health (Larchmt). 2005 Sep;14(7):595-608. doi: 10.1089/jwh.2005.14.595. J Womens Health (Larchmt). 2005. PMID: 16181016 Review.
References
-
- Bernard H. T. et al., “An international urogynecological association(IUGA)/international continence society (ICS) joint reporton the terminology for female pelvic floor dysfunction,” Neurourology Urodynamics, vol. 29, no. 1, pp. 4–20, 2010. - PubMed
-
- Park J., Bloom D., and McGuire E., “The guarding reflex revisited,” Brit. J. Urol., vol. 80, no. 6, pp. 940–945, 1997. - PubMed
-
- Lee U. J. et al., “Prevalence of urinary incontinence among a nationally representative sample of women, 2005-2016: Findings from the urologic diseases in America project,” J. Urol., vol. 205, no. 6, pp. 1718–1724, 2021. - PubMed
LinkOut - more resources
Full Text Sources

