How Rigid Are Anthranilamide Molecular Electrets?
- PMID: 39564657
- PMCID: PMC11831674
- DOI: 10.1021/acs.jpcb.4c04103
How Rigid Are Anthranilamide Molecular Electrets?
Abstract
As important as molecular electrets are for electronic materials and devices, conformational fluctuations strongly impact their macrodipoles and intrinsic properties. Herein, we employ molecular dynamics (MD) simulations with the polarizable charge equilibrium (PQEq) method to investigate the persistence length (LP) of molecular electrets composed of anthranilamide (Aa) residues. The PQEq-MD dissipates the accepted static notions about Aa macromolecules, and LP represents the shortest Aa rigid segments. The classical model with a single LP value does not describe these oligomers. Introducing multiple LP values for the same macromolecule follows the observed trends and discerns the enhanced rigidity in their middle sections from the reduced stiffness at their terminal regions. Furthermore, LP distinctly depends on solvent polarity. The Aa oligomers maintain extended conformations in nonpolar solvents with LP exceeding 4 nm, while in polar media, increased conformational fluctuations reduce LP to about 2 nm. These characteristics set key guidelines about the utility of Aa conjugates for charge-transfer systems within organic electronics and energy engineering.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
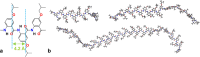
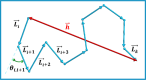
 . The angle θi,j depicts the direction that a vector
. The angle θi,j depicts the direction that a vector  makes with a reference vector
makes with a reference vector  . The vector, connecting the head of
. The vector, connecting the head of  and the tail of
and the tail of  , represents the end-to-end distance when i = 1, i.e., the first residue of the polymer, and k = n, i.e., the last residue of a polymer composed of n units. The length of each vector,
, represents the end-to-end distance when i = 1, i.e., the first residue of the polymer, and k = n, i.e., the last residue of a polymer composed of n units. The length of each vector,  , is the same, designated with LB. The differences between the lengths
of the shown arrows originate from the projection of the three-dimensional
arrangement of these vectors in the plane of their two-dimensional
representation of the figure.
, is the same, designated with LB. The differences between the lengths
of the shown arrows originate from the projection of the three-dimensional
arrangement of these vectors in the plane of their two-dimensional
representation of the figure.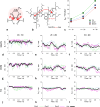

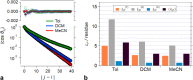
 of Aa oligomers for solvents with different
polarity. (a) Representative correlation decays for Tol, DCM and MeCN,
along with the triexponential fits (eq 4) and the corresponding residuals from these SCF analysis.
The θi,j values
are extracted from the MD simulation for the electret surrounded by
explicitly introduced Tol, DCM and MeCN solvents, and the corresponding
cos θi,j are averaged
of all the segmented frames from the 1 ns MD simulations. (b) The LP of the Aa electrets obtained
from the triexponential SCF fits and assigned to LP(0), LP(T) and LP(K), based on the patterns from
the GF analysis (see
of Aa oligomers for solvents with different
polarity. (a) Representative correlation decays for Tol, DCM and MeCN,
along with the triexponential fits (eq 4) and the corresponding residuals from these SCF analysis.
The θi,j values
are extracted from the MD simulation for the electret surrounded by
explicitly introduced Tol, DCM and MeCN solvents, and the corresponding
cos θi,j are averaged
of all the segmented frames from the 1 ns MD simulations. (b) The LP of the Aa electrets obtained
from the triexponential SCF fits and assigned to LP(0), LP(T) and LP(K), based on the patterns from
the GF analysis (see  provides a single value for the LP considering the contributions
from all the components.
provides a single value for the LP considering the contributions
from all the components.Similar articles
-
How Permanent Are the Permanent Macrodipoles of Anthranilamide Bioinspired Molecular Electrets?J Am Chem Soc. 2024 Feb 28;146(8):5162-5172. doi: 10.1021/jacs.3c10525. Epub 2024 Jan 16. J Am Chem Soc. 2024. PMID: 38226894 Free PMC article.
-
Theoretical design of bioinspired macromolecular electrets based on anthranilamide derivatives.Biotechnol Prog. 2009 Jul-Aug;25(4):915-22. doi: 10.1002/btpr.189. Biotechnol Prog. 2009. PMID: 19452534
-
Anthranilamides as bioinspired molecular electrets: experimental evidence for a permanent ground-state electric dipole moment.J Org Chem. 2013 Mar 1;78(5):1994-2004. doi: 10.1021/jo301942g. Epub 2013 Jan 3. J Org Chem. 2013. PMID: 23270467
-
Lid opening and conformational stability of T1 Lipase is mediated by increasing chain length polar solvents.PeerJ. 2017 May 18;5:e3341. doi: 10.7717/peerj.3341. eCollection 2017. PeerJ. 2017. PMID: 28533982 Free PMC article.
-
Synergistic Approach of Ultrafast Spectroscopy and Molecular Simulations in the Characterization of Intramolecular Charge Transfer in Push-Pull Molecules.Molecules. 2020 Jan 20;25(2):430. doi: 10.3390/molecules25020430. Molecules. 2020. PMID: 31968694 Free PMC article. Review.
References
-
- Fahlman M.; Fabiano S.; Gueskine V.; Simon D.; Berggren M.; Crispin X. Interfaces in organic electronics. Nat. Rev. Mater. 2019, 4, 627–650. 10.1038/s41578-019-0127-y. - DOI
LinkOut - more resources
Full Text Sources
Research Materials

