Comparative Efficacy and Safety of Baricitinib Against Traditional Therapies in Severe Alopecia Areata: A Retrospective Cohort Study
- PMID: 39564906
- PMCID: PMC11845945
- DOI: 10.1111/jocd.16666
Comparative Efficacy and Safety of Baricitinib Against Traditional Therapies in Severe Alopecia Areata: A Retrospective Cohort Study
Abstract
Introduction: Alopecia areata is a common autoimmune disease which results in reversible hair loss. Janus kinase inhibitors are prescribed for severe alopecia areata with encouraging results. There are no studies comparing the efficacy and safety of Janus kinase inhibitors to traditional treatment options, such as topical immunomodulators and traditional immunosuppressants.
Aims: To retrospectively compare the efficacy and safety of baricitinib, an approved Janus kinase inhibitor, to other treatments for severe AA during a 6-month treatment period.
Materials/methods: We included patients with newly presenting, relapsing or treatment-resistant alopecia areata with Severity of Alopecia Tool (SALT) score ≥ 50, for the period between July 2021 and July 2023. Medical histories were reviewed and possible side effects were recorded. Primary endpoints were SALT ≤ 20 and SALT ≤ 10 after 6 months of treatment.
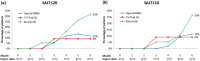
Results: Seventy-five patients (53 females) were divided into three groups: topical immunomodulators (51 patients); baricitinib (19 patients); and a group receiving pulsed intramuscular corticosteroids or traditional immunosuppressants (11 patients). Twenty-one patients received more than one treatment options within 2 years. After 6 months, the baricitinib group showed superior efficacy with 32% and 26% of patients achieving SALT ≤ 20 and SALT ≤ 10, compared to 12% and 9% in both other groups. Baricitinib demonstrated better secondary outcomes (50% and 90% reduction from initial SALT scores). All treatments exhibited mild-to-moderate and expected side effects. Weight gain, which had not been reported in clinical trials for alopecia areata, was observed in three baricitinib-treated patients.
Conclusion: Baricitinib was superior to traditional treatments for severe alopecia areata after 6 months. Weight gain concerned 16% of patients receiving baricitinib.
Keywords: JAK inhibitors; alopecia areata; baricitinib; baricitinib efficacy; baricitinib safety.
© 2024 The Author(s). Journal of Cosmetic Dermatology published by Wiley Periodicals LLC.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

Similar articles
-
Switching JAK inhibitors: evaluating baricitinib's effectiveness in alopecia areata after tofacitinib failure.Arch Dermatol Res. 2025 Feb 25;317(1):491. doi: 10.1007/s00403-025-04035-y. Arch Dermatol Res. 2025. PMID: 39998595
-
Baricitinib for the treatment of severe alopecia areata: results from a 52-week multicenter retrospective real-world study.J Dermatolog Treat. 2025 Dec;36(1):2444494. doi: 10.1080/09546634.2024.2444494. Epub 2025 Jan 6. J Dermatolog Treat. 2025. PMID: 39761973
-
Predictive factors for treatment responses to baricitinib in severe alopecia areata: A retrospective, multivariate analysis of 70 cases from a single center.J Dermatol. 2025 Apr;52(4):701-711. doi: 10.1111/1346-8138.17641. Epub 2025 Jan 27. J Dermatol. 2025. PMID: 39868612
-
Comparative efficacy and safety of systemic steroids, oral JAK inhibitors and Contact Immunotherapy in the Treatment of severe alopecia areata: a systematic review and network meta-analysis.Arch Dermatol Res. 2024 Jul 23;316(7):483. doi: 10.1007/s00403-024-03177-9. Arch Dermatol Res. 2024. PMID: 39042154
-
A Review of JAK Inhibitors for Treatment of Alopecia Areata in the Military Health Care System.Mil Med. 2025 Jan 16;190(1-2):e67-e73. doi: 10.1093/milmed/usae292. Mil Med. 2025. PMID: 38850223 Review.
Cited by
-
Efficacy of Janus Kinase Inhibitors in Alopecia in Jordanian Patients: A Retrospective Cohort Study.Cureus. 2025 May 18;17(5):e84321. doi: 10.7759/cureus.84321. eCollection 2025 May. Cureus. 2025. PMID: 40530228 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

