Extramacrochaetae regulates Notch signaling in the Drosophila eye through non-apoptotic caspase activity
- PMID: 39564985
- PMCID: PMC11578588
- DOI: 10.7554/eLife.91988
Extramacrochaetae regulates Notch signaling in the Drosophila eye through non-apoptotic caspase activity
Abstract
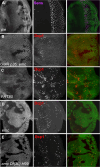
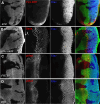
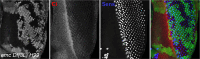
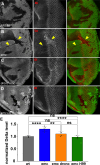
Many cell fate decisions are determined transcriptionally. Accordingly, some fate specification is prevented by Inhibitor of DNA-binding (Id) proteins that interfere with DNA binding by master regulatory transcription factors. We show that the Drosophila Id protein Extra macrochaetae (Emc) also affects developmental decisions by regulating caspase activity. Emc, which prevents proneural bHLH transcription factors from specifying neural cell fate, also prevents homodimerization of another bHLH protein, Daughterless (Da), and thereby maintains expression of the Death-Associated Inhibitor of Apoptosis (diap1) gene. Accordingly, we found that multiple effects of emc mutations on cell growth and on eye development were all caused by activation of caspases. These effects included acceleration of the morphogenetic furrow, failure of R7 photoreceptor cell specification, and delayed differentiation of non-neuronal cone cells. Within emc mutant clones, Notch signaling was elevated in the morphogenetic furrow, increasing morphogenetic furrow speed. This was associated with caspase-dependent increase in levels of Delta protein, the transmembrane ligand for Notch. Posterior to the morphogenetic furrow, elevated Delta cis-inhibited Notch signaling that was required for R7 specification and cone cell differentiation. Growth inhibition of emc mutant clones in wing imaginal discs also depended on caspases. Thus, emc mutations reveal the importance of restraining caspase activity even in non-apoptotic cells to prevent abnormal development, in the Drosophila eye through effects on Notch signaling.
Keywords: D. melanogaster; Delta; Drosophila eye; ID protein; caspase; developmental biology; extramacrochaetae; non-apoptotic caspase.
© 2023, Nair and Baker.
Conflict of interest statement
SN, NB No competing interests declared
Figures








Update of
-
Extramacrochaetae regulates Notch signaling in the Drosophila eye through non-apoptotic caspase activity.bioRxiv [Preprint]. 2024 Oct 28:2023.10.04.560841. doi: 10.1101/2023.10.04.560841. bioRxiv. 2024. Update in: Elife. 2024 Nov 20;12:RP91988. doi: 10.7554/eLife.91988. PMID: 39131389 Free PMC article. Updated. Preprint.
Similar articles
-
Extramacrochaetae regulates Notch signaling in the Drosophila eye through non-apoptotic caspase activity.bioRxiv [Preprint]. 2024 Oct 28:2023.10.04.560841. doi: 10.1101/2023.10.04.560841. bioRxiv. 2024. Update in: Elife. 2024 Nov 20;12:RP91988. doi: 10.7554/eLife.91988. PMID: 39131389 Free PMC article. Updated. Preprint.
-
The Id protein Extramacrochaetae restrains the E protein Daughterless to regulate Notch, Rap1, and Sevenless within the R7 equivalence group of the Drosophila eye.Biol Open. 2024 Aug 15;13(8):bio060124. doi: 10.1242/bio.060124. Epub 2024 Aug 20. Biol Open. 2024. PMID: 39041866 Free PMC article.
-
The HLH protein Extramacrochaetae is required for R7 cell and cone cell fates in the Drosophila eye.Dev Biol. 2009 Mar 15;327(2):288-300. doi: 10.1016/j.ydbio.2008.11.037. Epub 2008 Dec 11. Dev Biol. 2009. PMID: 19118542 Free PMC article.
-
Apoptosis-induced compensatory proliferation. The Cell is dead. Long live the Cell!Trends Cell Biol. 2008 Oct;18(10):467-73. doi: 10.1016/j.tcb.2008.08.001. Epub 2008 Sep 4. Trends Cell Biol. 2008. PMID: 18774295 Free PMC article. Review.
-
Pattern formation in the Drosophila eye.Curr Opin Genet Dev. 2007 Aug;17(4):309-13. doi: 10.1016/j.gde.2007.05.001. Epub 2007 Jul 6. Curr Opin Genet Dev. 2007. PMID: 17618111 Free PMC article. Review.
Cited by
-
Transcriptional Repression of reaper by Stand Still Safeguards Female Germline Development in Drosophila.bioRxiv [Preprint]. 2025 Jun 3:2025.05.17.654630. doi: 10.1101/2025.05.17.654630. bioRxiv. 2025. PMID: 40501627 Free PMC article. Preprint.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

