Durlobactam to boost the clinical utility of standard of care β-lactams against Mycobacterium abscessus lung disease
- PMID: 39565116
- PMCID: PMC11784023
- DOI: 10.1128/aac.01046-24
Durlobactam to boost the clinical utility of standard of care β-lactams against Mycobacterium abscessus lung disease
Abstract
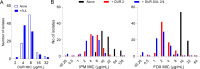
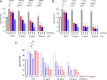
β-Lactams present several desirable pharmacodynamic features leading to the rapid eradication of many bacterial pathogens. Imipenem (IPM) and cefoxitin (FOX) are injectable β-lactams recommended during the intensive treatment phase of pulmonary infections caused by Mycobacterium abscessus (Mab). However, their potency against Mab is many-fold lower than against Gram-positive and Gram-negative pathogens for which they were optimized, putting into question their clinical utility. Here, we show that adding the recently approved durlobactam-sulbactam (DUR-SUL) pair to either IPM or FOX achieves growth inhibition, bactericidal, and cytolytic activity at concentrations that are within those achieved in patients and below the clinical breakpoints established for each agent. Synergies between DUR-SUL and IPM or FOX were confirmed across a large panel of clinical isolates. Through in vitro resistant mutant selection, we also show that adding DUR-SUL abrogates acquired resistance to IPM and FOX. Since the use of β-lactam injectables is firmly grounded in clinical practice during the intensive treatment phase of Mab pulmonary disease, their potentiation by FDA-approved DUR-SUL to bring minimum inhibitory concentration distributions within achievable concentration ranges could offer significant short-term benefits to patients, while novel β-lactam combinations are optimized specifically against Mab pulmonary infections, for which no reliable cure exists.
Keywords: MmpL11; MspA; Mycobacterium abscessus; RshA; drug resistance; lung infection; β-lactams.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Synergistic Interactions of Indole-2-Carboxamides and β-Lactam Antibiotics against Mycobacterium abscessus.Antimicrob Agents Chemother. 2020 Apr 21;64(5):e02548-19. doi: 10.1128/AAC.02548-19. Print 2020 Apr 21. Antimicrob Agents Chemother. 2020. PMID: 32041716 Free PMC article.
-
Dual β-Lactam Combinations Highly Active against Mycobacterium abscessus Complex In Vitro.mBio. 2019 Feb 12;10(1):e02895-18. doi: 10.1128/mBio.02895-18. mBio. 2019. PMID: 30755518 Free PMC article.
-
Effect of β-lactamase production and β-lactam instability on MIC testing results for Mycobacterium abscessus.J Antimicrob Chemother. 2017 Nov 1;72(11):3070-3078. doi: 10.1093/jac/dkx284. J Antimicrob Chemother. 2017. PMID: 28961987
-
Sulbactam-durlobactam: a β-lactam/β-lactamase inhibitor combination targeting Acinetobacter baumannii.Future Microbiol. 2024;19(7):563-576. doi: 10.2217/fmb-2023-0248. Epub 2024 Mar 1. Future Microbiol. 2024. PMID: 38426849 Free PMC article. Review.
-
Dual β-lactams for the treatment of Mycobacterium abscessus: a review of the evidence and a call to act against an antibiotic nightmare.J Antimicrob Chemother. 2024 Nov 4;79(11):2731-2741. doi: 10.1093/jac/dkae288. J Antimicrob Chemother. 2024. PMID: 39150384 Free PMC article. Review.
References
-
- Kwak N, Dalcolmo MP, Daley CL, Eather G, Gayoso R, Hasegawa N, Jhun BW, Koh W-J, Namkoong H, Park J, Thomson R, van Ingen J, Zweijpfenning SMH, Yim J-J. 2019. M ycobacterium abscessus pulmonary disease: individual patient data meta-analysis. Eur Respir J 54:1801991. doi:10.1183/13993003.01991-2018 - DOI - PubMed
-
- Ferro BE, Srivastava S, Deshpande D, Pasipanodya JG, van Soolingen D, Mouton JW, van Ingen J, Gumbo T. 2016. Failure of the amikacin, cefoxitin, and clarithromycin combination regimen for treating pulmonary Mycobacterium abscessus infection. Antimicrob Agents Chemother 60:6374–6376. doi:10.1128/AAC.00990-16 - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

