Cigarette smoke-induced disordered microbiota aggravates the severity of influenza A virus infection
- PMID: 39565120
- PMCID: PMC11651097
- DOI: 10.1128/msystems.00790-24
Cigarette smoke-induced disordered microbiota aggravates the severity of influenza A virus infection
Abstract
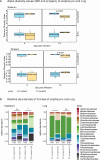
Cigarette smoke (CS) promotes the development of chronic pulmonary disease and has been associated with increased risk for influenza-related illness. Here, we directly addressed the impact of CS disordered microbiota on the severity of influenza A virus (IAV) infection. Specific and opportunistic pathogen-free (SOPF) C57BL/6J mice were exposed to CS or room air (RA) for 5.5 months. Each exposed mouse was then cohoused with a group of recipient germ-free (GF) mice for 1 month for microbial transfer. Colonized GF mice were then infected intranasally with IAV and disease development was monitored. Upper and lower airway and fecal microbiota were longitudinally investigated by 16S rRNA gene sequencing and bacterial cultures in donor and recipient mice. The bacterial family Streptococcaceae accounted for the largest difference between CS- and RA-exposed microbiota in the oropharynx. Analysis of the oropharynx and fecal microbiota indicated an efficient transfer to coprophagic recipient mice, which replicated the differences in microbiota composition observed in donor mice. Subsequent IAV infection revealed significantly higher weight loss for CS microbiota recipient mice at 8-10 days post infection (dpi) compared to control recipient mice. In addition, H1N1 infection inflicted substantial changes in the microbiota composition, especially at days 4 and 8 after infection. In conclusion, mice with a CS-associated microbiota suffer from higher disease severity upon IAV infection compared to mice colonized with a normal SOPF microbiota. Our data suggest that independently of CS exposure and concomitant structural lung damage, microbial distortion due to CS exposure may impact the severity of IAV disease course.IMPORTANCEIt has been reported that chronic exposure to CS is associated with a disordered microbiota composition. In this study, we colonized germ-free (GF) mice with the microbiota from SOPF mice which were chronically exposed to CS or RA. This allowed disentangling the effect of the disordered microbiota from the immune-modulating effects of actual CS exposure. We observed a successful transfer of the microbiotas after cohousing including specific microbiota differences induced by CS exposure in formerly GF mice, which were never exposed to CS. We then investigated the effects of IAV infection on the disease course and microbiotas of formerly GF mice. We found that mice with CS-associated microbiota reveal worse disease course compared to the control group. We hypothesize that CS-induced disordering of the microbiota may, indeed, impact the severity of influenza A disease.
Keywords: H1N1 infection; airways; cigarette smoke; feces; germ free mice; microbiota.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






References
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
