Secukinumab Reduces Psoriasis-associated Pruritus and Regenerates the Cutaneous Nerve Architecture: Results from PSORITUS a Doubleblind, Placebo-controlled, Randomized Withdrawal Phase IIIb Study
- PMID: 39565228
- PMCID: PMC11600607
- DOI: 10.2340/actadv.v104.40737
Secukinumab Reduces Psoriasis-associated Pruritus and Regenerates the Cutaneous Nerve Architecture: Results from PSORITUS a Doubleblind, Placebo-controlled, Randomized Withdrawal Phase IIIb Study
Abstract
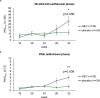
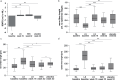
The occurrence of pruritus in psoriasis was previously underestimated but is a significant burden. Secukinumab (SEC), a monoclonal anti-interleukin-17A antibody, efficiently controls signs of psoriasis, but the effect on pruritus and cutaneous neuroanatomy remained unknown. The primary objective of this study (NCT02362789) was to evaluate the superiority of SEC treatment vs placebo on pruritus intensity (visual analogue scale; VAS). Furthermore, the treatment-dependent course of pruritus in association with absolute Psoriasis Area Severity Index (PASI) score, as well as cutaneous histopathology and neuroanatomy, was assessed. Open-label SEC 300 mg s.c. was administered regularly until week 16. Patients who reached a ≥ 98% PASI reduction (PASI ≥ 98) were randomized to receive either placebo or SEC up to week 32. Punch biopsies were collected from lesional psoriatic (baseline, weeks 16 and 32) and non-lesional (baseline) skin for histopathological and neuroanatomical analyses. VAS scores improved significantly after open-label SEC treatment but relapsed upon placebo (29.92 ± 33.8) compared with SEC (12.30 ± 22.6; p = 0.036). After SEC-dependent improvement in PASI, histopathology, marker expression and neuroanatomy, relapse was observed with treatment discontinuation in all parameters except neuroanatomy. SEC was superior to placebo by efficiently controlling reduced pruritus intensity, clinically normalizing skin lesions, and reversing histopathological abnormalities. The neuroanatomy recovered upon SEC and remained stable even after withdrawal.
Conflict of interest statement
SST was a speaker and/or consultant and/or investigator and/or has received research funding from Abbvie, Almirall, Beiersdorf, BMS, Clexio, Eli Lilly, FomF, Galderma, German Research Foundation (DFG), Integrity CE, Kiniksa, Leo Pharma, L’Oréal, MEDahead, Moroscience, NACCME, Novartis, Omnicuris, P.G. Unna Academy, Pfizer, Sanofi, TouchIME, UCB, Vifor, and WebMD. MPP is an investigator for Allakos, Celldex Therapeutics, Incyte, Sanofi, and Trevi Therapeutics; and has received consultant and/or speaker honoraria and/or travel fees from AbbVie, Beiersdorf, Eli Lilly, GA2LEN, Galderma, Menlo Therapeutics, Novartis, P.G. Unna Academy, Sanofi, and Trevi Therapeutics. KL was a speaker and/or consultant and/or investigator and/or has received research funding from Amgen, Biogen, Dr. Wolff Arzneimittel, Estée Lauder, the German Research Foundation (DFG), Janssen, Kiniksa, Leo Pharma, Novartis, and TEVA. DB was an employee of Novartis Pharma GmbH Germany. NM is an employee of Novartis Pharma GmbH Germany. MR is an employee of Novartis Pharma AG Switzerland. All other authors have no conflicts of interest to declare.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

