De novo missense variants in the PP2A regulatory subunit PPP2R2B in a neurodevelopmental syndrome: potential links to mitochondrial dynamics and spinocerebellar ataxias
- PMID: 39565297
- PMCID: PMC11780858
- DOI: 10.1093/hmg/ddae166
De novo missense variants in the PP2A regulatory subunit PPP2R2B in a neurodevelopmental syndrome: potential links to mitochondrial dynamics and spinocerebellar ataxias
Abstract
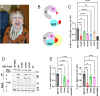
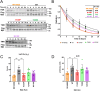
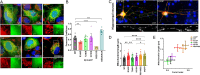
The heterotrimeric protein phosphatase 2A (PP2A) complex catalyzes about half of Ser/Thr dephosphorylations in eukaryotic cells. A CAG repeat expansion in the neuron-specific protein PP2A regulatory subunit PPP2R2B gene causes spinocerebellar ataxia type 12 (SCA12). We established five monoallelic missense variants in PPP2R2B (four confirmed as de novo) as a cause of intellectual disability with developmental delay (R149P, T246K, N310K, E37K, I427T). In addition to moderate to severe intellectual disability and developmental delay, affected individuals presented with seizures, microcephaly, aggression, hypotonia, as well as broad-based or stiff gait. We used biochemical and cellular assays, including a novel luciferase complementation assay to interrogate PP2A holoenzyme assembly and activity, as well as deregulated mitochondrial dynamics as possible pathogenic mechanisms. Cell-based assays documented impaired ability of PPP2R2B missense variants to incorporate into the PP2A holoenzyme, localize to mitochondria, induce fission of neuronal mitochondria, and dephosphorylate the mitochondrial fission enzyme dynamin-related protein 1. AlphaMissense-based pathogenicity prediction suggested that an additional seven unreported missense variants may be pathogenic. In conclusion, our studies identify loss-of-function at the PPP2R2B locus as the basis for syndromic intellectual disability with developmental delay. They also extend PPP2R2B-related pathologies from neurodegenerative (SCA12) to neurodevelopmental disorders and suggests that altered mitochondrial dynamics may contribute to mechanisms.
Keywords: cerebellar ataxias; dynamin-related protein 1; mitochondrial dynamics; neurodevelopmental disorders; protein phosphatase 2A.
© The Author(s) 2024. Published by Oxford University Press.
Figures

References
-
- Verbinnen I, Vaneynde P, Reynhout S. et al. Protein phosphatase 2A (PP2A) mutations in brain function, development, and neurologic disease. Biochem Soc Trans 2021;49:1567–1588. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

