KCTD10 p.C124W variant contributes to schizophrenia by attenuating LLPS-mediated synapse formation
- PMID: 39565307
- PMCID: PMC11621769
- DOI: 10.1073/pnas.2400464121
KCTD10 p.C124W variant contributes to schizophrenia by attenuating LLPS-mediated synapse formation
Abstract
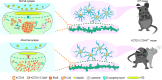
KCTD10, a member of the potassium channel tetramerization domain (KCTD) family, is implicated in neuropsychiatric disorders and functions as a substrate recognition component within the RING-type ubiquitin ligase complex. A rare de novo variant of KCTD10, p.C124W, was identified in schizophrenia cases, yet its underlying pathogenesis remains unexplored. Here, we demonstrate that heterozygous KCTD10 C124W mice display pronounced synaptic abnormalities and exhibit schizophrenia-like behaviors. Mechanistically, we reveal that KCTD10 undergoes liquid-liquid phase separation (LLPS), a process orchestrated by its intrinsically disordered region (IDR). p.C124W mutation disrupts this LLPS capability, leading to diminished degradation of RHOB and subsequent excessive accumulation in the postsynaptic density fractions. Notably, neither IDR deletion nor p.C124W mutation in KCTD10 mitigates the synaptic abnormalities caused by Kctd10 deficiency. Thus, our findings implicate that LLPS may be associated with the pathogenesis of KCTD10-associated brain disorders and highlight the potential of targeting RHOB as a therapeutic strategy for diseases linked to mutations in KCTD10 or RHOB.
Keywords: KCTD10; liquid–liquid phase separation (LLPS); neuropsychiatric disorder; synaptic abnormalities.
Conflict of interest statement
Competing interests statement:The authors declare no competing interest.
Figures
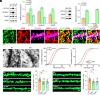



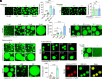
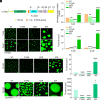
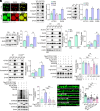
References
-
- Zhang H., et al. , Liquid-liquid phase separation in biology: Mechanisms, physiological functions and human diseases. Sci. China Life Sci. 63, 953–985 (2020). - PubMed
-
- Feng Z., Chen X., Zeng M., Zhang M., Phase separation as a mechanism for assembling dynamic postsynaptic density signalling complexes. Curr. Opin. Neurobiol. 57, 1–8 (2019). - PubMed
-
- Chen X., Wu X., Wu H., Zhang M., Phase separation at the synapse. Nat. Neurosci. 23, 301–310 (2020). - PubMed
MeSH terms
Substances
Grants and funding
- 32070980/MOST | National Natural Science Foundation of China (NSFC)
- 32271026/MOST | National Natural Science Foundation of China (NSFC)
- 81901168/MOST | National Natural Science Foundation of China (NSFC)
- 32330038/MOST | National Natural Science Foundation of China (NSFC)
- 32394030/MOST | National Natural Science Foundation of China (NSFC)
LinkOut - more resources
Full Text Sources
Medical

