Use of Biologic or Targeted Synthetic Disease-Modifying Antirheumatic Drugs and Cancer Risk
- PMID: 39565623
- PMCID: PMC11579790
- DOI: 10.1001/jamanetworkopen.2024.46336
Use of Biologic or Targeted Synthetic Disease-Modifying Antirheumatic Drugs and Cancer Risk
Abstract
Importance: The Oral Rheumatoid Arthritis Trial Surveillance demonstrated an increased cancer risk among patients with rheumatoid arthritis (RA) taking tofacitinib compared with those taking tumor necrosis factor inhibitors (TNFis). Although international cohort studies have compared cancer outcomes between TNFis, non-TNFi drugs, and Janus kinase inhibitor (JAKis), their generalizability to US patients with RA is limited.
Objective: To assess the comparative safety of TNFis, non-TNFi drugs, and JAKis among US patients with RA (ie, the cancer risk associated with the use of these drugs among these patients).
Design, setting, and participants: This retrospective cohort study used US administrative claims data from Merative Marketscan Research Databases from November 1, 2012, to December 31, 2021. Follow-up occurred up to 2 years after initiation of biologic or targeted synthetic disease-modifying antirheumatic drugs (DMARDs). Participants included individuals aged 18 to 64 years with RA, identified using at least 2 RA International Classification of Diseases, Ninth Revision or International Statistical Classification of Diseases and Related Health Problems, Tenth Revision diagnostic codes on or before the date of TNFi, non-TNFi, or JAKi initiation ("index date"). Statistical analysis took place from June 2022 to September 2024.
Exposures: New initiations of TNFis, abatacept, interleukin 6 inhibitors (IL-6is), rituximab, or JAKis. Individuals could contribute person-time to more than 1 treatment exposure if treatment escalation mimicked typical clinical practice but were censored if they switched to a previously trialed medication class.
Main outcomes and measures: Incident cancer, excluding nonmelanoma skin cancer, after at least 90 days and within 2 years of initiation of biologic or targeted synthetic DMARDs. Outcomes were associated with the most recent drug exposure.
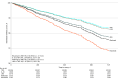
Results: Of the 25 305 individuals who initiated treatment and who met the inclusion criteria, most were female (19 869 [79%]), had a median age of 50 years (IQR, 42-56 years), and were from the South US (12 516 [49%]). Of a total 27 661 drug exposures, drug initiations consisted of 20 586 TNFi exposures (74%), 2570 JAKi exposures (9%), 2255 abatacept exposures (8%), 1182 rituximab exposures (4%), and 1068 IL-6i exposures (4%). Multivariable Cox proportional hazards regression analysis showed that rituximab was associated with a higher risk of incident cancer compared with TNFis (hazard ratio [HR], 1.91; 95% CI, 1.17-3.14), followed by abatacept (HR, 1.47; 95% CI, 1.03-2.11), and JAKis (HR, 1.36; 95% CI, 0.94-1.96).
Conclusions and relevance: In this cohort study of individuals with RA and new biologic or targeted synthetic DMARD exposures, individuals initiating rituximab, abatacept, and JAKis demonstrated higher incidence rates and statistically significantly increased risks of incident cancers compared with those initiating TNFis in the first 2 years after initiation of biologic or targeted synthetic DMARDs. Given the limitations of administrative claims data and confounding by indication, it is likely that these patients may have a higher disease burden, resulting in channeling bias. To better understand these associations, larger studies with longer follow-up time are needed.
Conflict of interest statement
Figures


References
-
- Cohen SB, Emery P, Greenwald MW, et al. ; REFLEX Trial Group . Rituximab for rheumatoid arthritis refractory to anti–tumor necrosis factor therapy: results of a multicenter, randomized, double-blind, placebo-controlled, phase III trial evaluating primary efficacy and safety at twenty-four weeks. Arthritis Rheum. 2006;54(9):2793-2806. doi:10.1002/art.22025 - DOI - PubMed
-
- Porter D, van Melckebeke J, Dale J, et al. . Tumour necrosis factor inhibition versus rituximab for patients with rheumatoid arthritis who require biological treatment (ORBIT): an open-label, randomised controlled, non-inferiority, trial. Lancet. 2016;388(10041):239-247. doi:10.1016/S0140-6736(16)00380-9 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

