Alternative LDL Cholesterol-Lowering Strategy vs High-Intensity Statins in Atherosclerotic Cardiovascular Disease: A Systematic Review and Individual Patient Data Meta-Analysis
- PMID: 39565634
- PMCID: PMC11579890
- DOI: 10.1001/jamacardio.2024.3911
Alternative LDL Cholesterol-Lowering Strategy vs High-Intensity Statins in Atherosclerotic Cardiovascular Disease: A Systematic Review and Individual Patient Data Meta-Analysis
Abstract
Importance: In patients with atherosclerotic cardiovascular disease (ASCVD), intensive lowering of low-density lipoprotein (LDL) cholesterol levels with high-intensity statins is generally recommended. However, alternative approaches considering statin-related adverse effects and intolerance are needed.
Objective: To compare the long-term efficacy and safety of an alternative LDL cholesterol-lowering strategy vs high-intensity statin strategy in patients with ASCVD in randomized clinical trials.
Data sources: PubMed, Embase, and other websites (ClinicalTrials.gov, European Society of Cardiology, tctMD) were systematically searched from inception to April 19, 2024.
Study selection: Randomized clinical trials comparing an alternative LDL cholesterol-lowering strategy vs a high-intensity statin strategy in patients with ASCVD, with presence of cardiovascular events as end points.
Data extraction and synthesis: Individual patient data were obtained from randomized clinical trials that met the prespecified eligibility criteria: RACING (Randomized Comparison of Efficacy and Safety of Lipid-Lowering With Statin Monotherapy vs Statin/Ezetimibe Combination for High-Risk Cardiovascular Disease) and LODESTAR (Low-Density Lipoprotein Cholesterol-Targeting Statin Therapy vs Intensity-Based Statin Therapy in Patients With Coronary Artery Disease). The moderate-intensity statin with ezetimibe combination therapy in the RACING trial and the treat-to-target strategy in the LODESTAR trial were classified as alternative LDL cholesterol-lowering strategies. The primary analysis was based on a 1-stage approach.
Main outcomes and measures: The primary end point was a 3-year composite of all-cause death, myocardial infarction, stroke, or coronary revascularization. The secondary end points comprised clinical efficacy and safety end points.
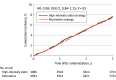
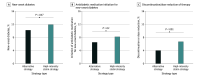
Results: Individual patient data from 2 trials including 8180 patients with ASCVD (mean [SD] age, 64.5 [9.8] years; 2182 [26.7%] female; 5998 male [73.3%]) were analyzed. The rate of the primary end point did not differ between the alternative strategy and high-intensity statin strategy groups (7.5% [304 of 4094] vs 7.7% [310 of 4086]; hazard ratio, 0.98; 95% CI, 0.84-1.15; P = .82). The mean (SD) LDL cholesterol level during treatment was 64.8 (19.0) mg/dL in the alternative strategy group and 68.5 (20.7) mg/dL in the high-intensity statin strategy group (P < .001). The alternative strategy group had a lower rate of new-onset diabetes (10.2% [271 of 2658] vs 11.9% [316 of 2656]; P = .047), initiation of antidiabetic medication for new-onset diabetes (6.5% [173 of 2658] vs 8.2% [217 of 2656]; P = .02), and intolerance-related discontinuation or dose reduction of assigned therapy (4.0% [163 of 4094] vs 6.7% [273 of 4086]; P < .001).
Conclusions and relevance: Results of this systematic review and individual patient data meta-analysis suggest that compared with a high-intensity statin strategy, the alternative LDL cholesterol-lowering strategy demonstrated comparable efficacy regarding 3-year death or cardiovascular events in patients with ASCVD, with an associated reduction in LDL cholesterol levels and risk for new-onset diabetes and intolerance.
Study registration: PROSPERO CRD42024532550.
Conflict of interest statement
Figures

References
-
- Grundy SM, Stone NJ, Bailey AL, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol: a report of the American College of Cardiology/American Heart Association task force on clinical practice guidelines. J Am Coll Cardiol. 2019;73(24):e285-e350. - PubMed
-
- Mach F, Baigent C, Catapano AL, et al. ; ESC Scientific Document Group . 2019 ESC/EAS guidelines for the management of dyslipidemias: lipid modification to reduce cardiovascular risk. Eur Heart J. 2020;41(1):111-188. - PubMed
-
- Cannon CP, Braunwald E, McCabe CH, et al. ; Pravastatin or Atorvastatin Evaluation and Infection Therapy-Thrombolysis in Myocardial Infarction 22 Investigators . Intensive vs moderate lipid lowering with statins after acute coronary syndromes. N Engl J Med. 2004;350(15):1495-1504. - PubMed
-
- LaRosa JC, Grundy SM, Waters DD, et al. ; Treating to New Targets (TNT) Investigators . Intensive lipid lowering with atorvastatin in patients with stable coronary disease. N Engl J Med. 2005;352(14):1425-1435. - PubMed
-
- Pedersen TR, Faergeman O, Kastelein JJ, et al. ; Incremental Decrease in End Points Through Aggressive Lipid Lowering (IDEAL) Study Group . High-dose atorvastatin vs usual-dose simvastatin for secondary prevention after myocardial infarction: the IDEAL study: a randomized controlled trial. JAMA. 2005;294(19):2437-2445. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

