Time from treatment initiation to HIV viral suppression in public care facilities in Brazil: A nationwide linked databases cohort
- PMID: 39565819
- PMCID: PMC11578461
- DOI: 10.1371/journal.pone.0305311
Time from treatment initiation to HIV viral suppression in public care facilities in Brazil: A nationwide linked databases cohort
Abstract
Objectives: To analyze the time between antiretroviral therapy (ART) initiation and the first HIV viral load (VL) test <40 copies-time to suppression (TS)-in a cohort of persons aged ≥15 years, between 2015-2018 in outpatient HIV care facilities of the Brazilian Unified Health System, as well as to analyze whether individual and facility characteristics accelerate or delay TS.
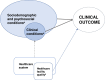
Methods: This was a cohort study with data from a linkage of national HIV databases, following a previously published procedure. Two types of variables were examined: individual-level (sex, age group, race/skin color, education, baseline CD4 cell count and VL, initial ART regimen, adherence, ART regimen change and number of VL tests until suppression) and facility-level (national and metropolitan region, caseload). Multilevel parametric accelerated failure time survival models were used. Fixed and random effects were analyzed through null, sociodemographic, combined sociodemographic and clinical, and facility-related variables, adjusted for the number of VL tests until suppression. Likelihood, interquartile range, and proportion of change in variance were used for comparisons.
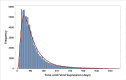
Results: Of 132,540 participants, 89.4% (114,696) achieved viral suppression: 20.8% within three months, and 56.4% within six months. Median TS was 161 days, varying from 31 to 1,426 days, depending on the time interval between initiation and VL testing. Among those who had VL testing within 66 days, median TS was 55 days. All individual and facility-related variables were associated with TS, explaining the 16.2% and 13.2% variability, respectively.
Conclusions: This was the first Brazilian nationwide cohort to analyze TS. It is also one of the largest operational cohorts globally to assess healthcare facility characteristics. The findings indicated that both individual and facility-related characteristics contribute to TS. Strengthening VL monitoring should be included as part of a coordinated effort to improve the quality of care provided for people living with HIV/AIDS in Brazil.
Copyright: © 2024 Nemes et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have declared that no competing interests exist
Figures

Similar articles
-
Linkage and retention in care and the time to HIV viral suppression and viral rebound - New York City.AIDS Care. 2015;27(2):260-7. doi: 10.1080/09540121.2014.959463. Epub 2014 Sep 22. AIDS Care. 2015. PMID: 25244545
-
HIV viral suppression and longevity among a cohort of children initiating antiretroviral therapy in Eastern Cape, South Africa.J Int AIDS Soc. 2018 Aug;21(8):e25168. doi: 10.1002/jia2.25168. J Int AIDS Soc. 2018. PMID: 30094952 Free PMC article.
-
Viral suppression among children and their caregivers living with HIV in western Kenya.J Int AIDS Soc. 2019 Apr;22(4):e25272. doi: 10.1002/jia2.25272. J Int AIDS Soc. 2019. PMID: 30983148 Free PMC article.
-
Adoption of routine virologic testing and predictors of virologic failure among HIV-infected children on antiretroviral treatment in western Kenya.PLoS One. 2018 Nov 9;13(11):e0200242. doi: 10.1371/journal.pone.0200242. eCollection 2018. PLoS One. 2018. PMID: 30412576 Free PMC article.
-
Viral load monitoring for people living with HIV in the era of test and treat: progress made and challenges ahead - a systematic review.BMC Public Health. 2022 Jun 16;22(1):1203. doi: 10.1186/s12889-022-13504-2. BMC Public Health. 2022. PMID: 35710413 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

