A potent proresolving mediator 17R-resolvin D2 from human macrophages, monocytes, and saliva
- PMID: 39565847
- PMCID: PMC11578181
- DOI: 10.1126/sciadv.adq4785
A potent proresolving mediator 17R-resolvin D2 from human macrophages, monocytes, and saliva
Abstract
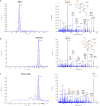
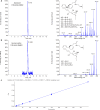
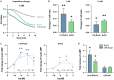
Production of specialized proresolving mediators (SPMs) during the resolution phase in the acute inflammatory response is key to orchestrating complete resolution. Here, we uncovered a trihydroxy resolvin in fresh human saliva. We identified and determined its complete stereochemistry as 7S,16R,17R-trihydroxy-4Z,8E,10Z,12E,14E,19Z-docosahexaenoic acid (17R-RvD2) using total organic synthesis and matching of physical properties. The 17R-RvD2 was produced by activated human M2-like macrophages, M1-like macrophages, and human peripheral blood monocytes. 17R-RvD2 displayed potent proresolving functions (picomolar to nanomolar). Topical application of 17R-RvD2 on mouse ear skin reduced neutrophilic infiltration (~50%). 17R-RvD2 increased M2 markers CD206 and CD163 on human monocyte-derived macrophages and enhanced efferocytosis of senescent red blood cells by M2-like macrophages (EC50 ~ 2.6 × 10-14 M). In addition, 17R-RvD2 activated the RvD2 receptor and was equipotent to its epimer RvD2. 17R-RvD2 also significantly increased phagocytosis of Escherichia coli by human neutrophils. Together, these results establish the complete stereochemistry and potent proresolving functions of the previously unknown 17R-RvD2.
Figures




References
-
- Libby P., Inflammation in atherosclerosis. Nature 420, 868–874 (2002). - PubMed
-
- Fredman G., Serhan C. N., Specialized pro-resolving mediators in vascular inflammation and atherosclerotic cardiovascular disease. Nat. Rev. Cardiol. 21, 808–823 (2024). - PubMed
-
- Samuelsson B., Dahlen S. E., Lindgren J. A., Rouzer C. A., Serhan C. N., Leukotrienes and lipoxins: Structures, biosynthesis, and biological effects. Science 237, 1171–1176 (1987). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

