Regulation of mitochondrial oxidative phosphorylation through tight control of cytochrome c oxidase in health and disease - Implications for ischemia/reperfusion injury, inflammatory diseases, diabetes, and cancer
- PMID: 39566165
- PMCID: PMC11617887
- DOI: 10.1016/j.redox.2024.103426
Regulation of mitochondrial oxidative phosphorylation through tight control of cytochrome c oxidase in health and disease - Implications for ischemia/reperfusion injury, inflammatory diseases, diabetes, and cancer
Abstract
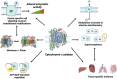
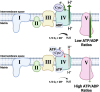
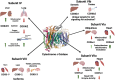
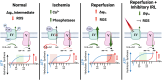
Mitochondria are essential to cellular function as they generate the majority of cellular ATP, mediated through oxidative phosphorylation, which couples proton pumping of the electron transport chain (ETC) to ATP production. The ETC generates an electrochemical gradient, known as the proton motive force, consisting of the mitochondrial membrane potential (ΔΨm, the major component in mammals) and ΔpH across the inner mitochondrial membrane. Both ATP production and reactive oxygen species (ROS) are linked to ΔΨm, and it has been shown that an imbalance in ΔΨm beyond the physiological optimal intermediate range results in excessive ROS production. The reaction of cytochrome c oxidase (COX) of the ETC with its small electron donor cytochrome c (Cytc) is the proposed rate-limiting step in mammals under physiological conditions. The rate at which this redox reaction occurs controls ΔΨm and thus ATP and ROS production. Multiple mechanisms are in place that regulate this reaction to meet the cell's energy demand and respond to acute stress. COX and Cytc have been shown to be regulated by all three main mechanisms, which we discuss in detail: allosteric regulation, tissue-specific isoforms, and post-translational modifications for which we provide a comprehensive catalog and discussion of their functional role with 55 and 50 identified phosphorylation and acetylation sites on COX, respectively. Disruption of these regulatory mechanisms has been found in several common human diseases, including stroke and myocardial infarction, inflammation including sepsis, and diabetes, where changes in COX or Cytc phosphorylation lead to mitochondrial dysfunction contributing to disease pathophysiology. Identification and subsequent targeting of the underlying signaling pathways holds clear promise for future interventions to improve human health. An example intervention is the recently discovered noninvasive COX-inhibitory infrared light therapy that holds promise to transform the current standard of clinical care in disease conditions where COX regulation has gone awry.
Copyright © 2024 The Authors. Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of competing interest Drs. Maik Hüttemann and Thomas Sanderson are co-founders of Mitovation, Inc., that develops IRL therapy for I/R injury applications. All other authors declare no potential conflict of interest.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

