A cell atlas foundation model for scalable search of similar human cells
- PMID: 39566551
- PMCID: PMC11864978
- DOI: 10.1038/s41586-024-08411-y
A cell atlas foundation model for scalable search of similar human cells
Abstract
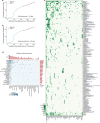
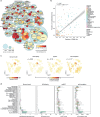
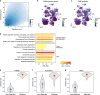
Single-cell RNA sequencing has profiled hundreds of millions of human cells across organs, diseases, development and perturbations to date. Mining these growing atlases could reveal cell-disease associations, identify cell states in unexpected tissue contexts and relate in vivo biology to in vitro models. These require a common measure of cell similarity across the body and an efficient way to search. Here we develop SCimilarity, a metric-learning framework to learn a unified and interpretable representation that enables rapid queries of tens of millions of cell profiles from diverse studies for cells that are transcriptionally similar to an input cell profile or state. We use SCimilarity to query a 23.4-million-cell atlas of 412 single-cell RNA-sequencing studies for macrophage and fibroblast profiles from interstitial lung disease1 and reveal similar cell profiles across other fibrotic diseases and tissues. The top scoring in vitro hit for the macrophage query was a 3D hydrogel system2, which we experimentally demonstrated reproduces this cell state. SCimilarity serves as a foundation model for single-cell profiles that enables researchers to query for similar cellular states across the human body, providing a powerful tool for generating biological insights from the Human Cell Atlas.
© 2024. The Author(s).
Conflict of interest statement
Competing interests: All of the authors are employees of Genentech or Roche. A.R. is a co-founder and equity holder of Celsius Therapeutics, an equity holder in Immunitas and, until 31 July 2020, was an scientific advisory board member of Thermo Fisher Scientific, Syros Pharmaceuticals, Neogene Therapeutics and Asimov. G.H., D.J.D., O.S., N.D., G.S., T.B., S.J.T., J.R.R., H.C.B., J.K., J.A.V.H. and A.R. have equity in Roche.
Figures










References
-
- Rood, J. E., Maartens, A., Hupalowska, A., Teichmann, S. A. & Regev, A. Impact of the Human Cell Atlas on medicine. Nat. Med.28, 2486–2496 (2022). - PubMed
-
- Cui, H. et al. scGPT: toward building a foundation model for single-cell multi-omics using generative AI. Nat. Methods21, 1470–1480 (2024). - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

