Studying Target-Engagement of Anti-Infectives by Solvent-Induced Protein Precipitation and Quantitative Mass Spectrometry
- PMID: 39566904
- PMCID: PMC11650766
- DOI: 10.1021/acsinfecdis.4c00417
Studying Target-Engagement of Anti-Infectives by Solvent-Induced Protein Precipitation and Quantitative Mass Spectrometry
Abstract
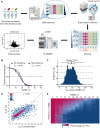
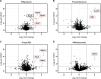
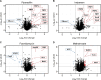
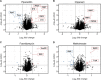
Antimicrobial resistance (AMR) poses a serious threat to global health. The rapid emergence of resistance contrasts with the slow pace of antimicrobial development, emphasizing the urgent need for innovative drug discovery approaches. This study addresses a critical bottleneck in early drug development by introducing integral solvent-induced protein precipitation (iSPP) to rapidly assess the target-engagement of lead compounds in extracts of pathogenic microorganisms under close-to-physiological conditions. iSPP measures the change in protein stability against solvent-induced precipitation in the presence of ligands. The iSPP method for bacteria builds upon established SPP procedures and features optimized denaturation gradients and minimized sample input amounts. The effectiveness of the iSPP workflow was initially demonstrated through a multidrug target-engagement study. Using quantitative mass spectrometry (LC-MS/MS), we successfully identified known drug targets of seven different antibiotics in cell extracts of four AMR-related pathogens: the three Gram-negative bacteria Escherichia coli, Klebsiella pneumoniae, Pseudomonas aeruginosa and the Gram-positive bacterium Staphylococcus aureus. The iSPP method was ultimately applied to demonstrate target-engagement of compounds derived from target-based drug discovery. We employed five small molecules targeting three enzymes in the 2-C-methyl-d-erythritol 4-phosphate (MEP) pathway─a promising focus for anti-infective drug development. The study showcases iSPP adaptability and efficiency in identifying anti-infective drug targets, advancing early-stage drug discovery against AMR.
Keywords: MEP pathway; antibiotics; mass spectrometry; proteomics; solvent-induced precipitation; target identification.
Conflict of interest statement
The authors declare the following competing financial interest(s): Hannes Hahne is co-founder and shareholder of OmicScouts GmbH, a proteomics and chemical proteomics-focused contract research organization.
Figures


Similar articles
-
Integral Solvent-Induced Protein Precipitation for Target-Engagement Studies in Plasmodium falciparum.ACS Infect Dis. 2024 Dec 13;10(12):4073-4086. doi: 10.1021/acsinfecdis.4c00418. Epub 2024 Dec 4. ACS Infect Dis. 2024. PMID: 39631773 Free PMC article.
-
Design SMAP29-LysPA26 as a Highly Efficient Artilysin against Pseudomonas aeruginosa with Bactericidal and Antibiofilm Activity.Microbiol Spectr. 2021 Dec 22;9(3):e0054621. doi: 10.1128/Spectrum.00546-21. Epub 2021 Dec 8. Microbiol Spectr. 2021. PMID: 34878337 Free PMC article.
-
A Whole-Cell Screen Identifies Small Bioactives That Synergize with Polymyxin and Exhibit Antimicrobial Activities against Multidrug-Resistant Bacteria.Antimicrob Agents Chemother. 2020 Feb 21;64(3):e01677-19. doi: 10.1128/AAC.01677-19. Print 2020 Feb 21. Antimicrob Agents Chemother. 2020. PMID: 31844003 Free PMC article.
-
Sulopenem: An Intravenous and Oral Penem for the Treatment of Urinary Tract Infections Due to Multidrug-Resistant Bacteria.Drugs. 2022 Apr;82(5):533-557. doi: 10.1007/s40265-022-01688-1. Epub 2022 Mar 16. Drugs. 2022. PMID: 35294769 Review.
-
[Antimicrobial drug therapy and microbial sensitivity tests: general concept of antibiotics].Rinsho Byori. 1997 Jul;Suppl 105:162-73. Rinsho Byori. 1997. PMID: 9379536 Review. Japanese. No abstract available.
Cited by
-
Target identification of natural products in cancer with chemical proteomics and artificial intelligence approaches.Cancer Biol Med. 2025 Jul 9;22(6):549-97. doi: 10.20892/j.issn.2095-3941.2025.0145. Cancer Biol Med. 2025. PMID: 40631551 Free PMC article. Review.
-
Integral Solvent-Induced Protein Precipitation for Target-Engagement Studies in Plasmodium falciparum.ACS Infect Dis. 2024 Dec 13;10(12):4073-4086. doi: 10.1021/acsinfecdis.4c00418. Epub 2024 Dec 4. ACS Infect Dis. 2024. PMID: 39631773 Free PMC article.
-
Extracellular vesicles as vital players in drug delivery: a focus on clinical disease treatment.Front Bioeng Biotechnol. 2025 May 14;13:1600227. doi: 10.3389/fbioe.2025.1600227. eCollection 2025. Front Bioeng Biotechnol. 2025. PMID: 40438295 Free PMC article. Review.
References
-
- Murray C. J.; Ikuta K. S.; Sharara F.; Swetschinski L.; Robles Aguilar G.; Gray A.; Han C.; Bisignano C.; Rao P.; Wool E.; et al. Global Burden Of Bacterial Antimicrobial Resistance In 2019: a Systematic Analysis. The Lancet 2022, 399 (10325), 629–655. 10.1016/S0140-6736(21)02724-0. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

