Targeting Hsp90α to inhibit HMGB1-mediated renal inflammation and fibrosis
- PMID: 39566909
- PMCID: PMC11882747
- DOI: 10.1111/cpr.13774
Targeting Hsp90α to inhibit HMGB1-mediated renal inflammation and fibrosis
Abstract
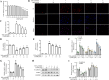
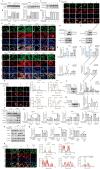
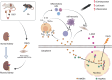
Renal fibrosis, a terminal manifestation of chronic kidney disease, is characterized by uncontrolled inflammatory responses, increased oxidative stress, tubular cell death, and imbalanced deposition of extracellular matrix. 5,2'-Dibromo-2,4',5'-trihydroxydiphenylmethanone (LM49), a polyphenol derivative synthesized by our group with excellent anti-inflammatory pharmacological properties, has been identified as a small-molecule inducer of extracellular matrix degradation. Nonetheless, the protective effects and mechanisms of LM49 on renal fibrosis remain unknown. Here, we report LM49 could effectively alleviate renal fibrosis and improve filtration function. Furthermore, LM49 significantly inhibited macrophage infiltration, pro-inflammatory cytokine production and oxidative stress. Interestingly, in HK-2 cells induced by tumour necrosis factor alpha under oxygen-glucose-serum deprivation conditions, LM49 treatment similarly yielded a reduced inflammatory response, elevated cellular viability and suppressed cell necrosis and epithelial-to-mesenchymal transition. Notably, LM49 prominently suppressed the high-mobility group box 1 (HMGB1) expression, nucleocytoplasmic translocation and activation. Mechanistically, drug affinity responsive target stability and cellular thermal shift assay confirmed that LM49 could interact with the target heat shock protein 90 alpha family class A member 1 (Hsp90α), disrupting the direct binding of Hsp90α to HMGB1 and inhibiting the nuclear export of HMGB1, thereby suppressing the inflammatory response, cell necrosis and fibrogenesis. Furthermore, molecular docking and molecular dynamic simulation revealed that LM49 occupied the N-terminal ATP pocket of Hsp90α. Collectively, our findings show that LM49 treatment can ameliorate renal fibrosis through inhibition of HMGB1-mediated inflammation and necrosis via binding to Hsp90α, providing strong evidence for its anti-inflammatory and anti-fibrotic actions.
© 2024 The Author(s). Cell Proliferation published by Beijing Institute for Stem Cell and Regenerative Medicine and John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures





Similar articles
-
GSK3β-dependent lysosome biogenesis: An effective pathway to mitigate renal fibrosis with LM49.Front Pharmacol. 2022 Sep 26;13:925489. doi: 10.3389/fphar.2022.925489. eCollection 2022. Front Pharmacol. 2022. PMID: 36225562 Free PMC article.
-
Therapeutic effects of 5,2'-dibromo-2,4',5'-trihydroxydiphenylmethanone (LM49) in an experimental rat model of acute pyelonephritis by immunomodulation and anti-inflammation.Int Immunopharmacol. 2018 Sep;62:155-164. doi: 10.1016/j.intimp.2018.07.001. Epub 2018 Jul 11. Int Immunopharmacol. 2018. PMID: 30007245
-
A novel marine halophenol derivative attenuates lipopolysaccharide-induced inflammation in RAW264.7 cells via activating phosphoinositide 3-kinase/Akt pathway.Pharmacol Rep. 2020 Aug;72(4):1021-1031. doi: 10.1007/s43440-019-00018-9. Epub 2020 Feb 28. Pharmacol Rep. 2020. PMID: 32112362
-
GSPE alleviates renal fibrosis by inhibiting the activation of C3/ HMGB1/ TGF-β1 pathway.Chem Biol Interact. 2020 Jan 25;316:108926. doi: 10.1016/j.cbi.2019.108926. Epub 2019 Dec 23. Chem Biol Interact. 2020. PMID: 31874164
-
Dapagliflozin ameliorates diabetic renal injury through suppressing the self-perpetuating cycle of inflammation mediated by HMGB1 feedback signaling in the kidney.Eur J Pharmacol. 2023 Mar 15;943:175560. doi: 10.1016/j.ejphar.2023.175560. Epub 2023 Feb 1. Eur J Pharmacol. 2023. PMID: 36736941
Cited by
-
Research progress on N6-methyladenosine and non-coding RNA in multiple myeloma.Discov Oncol. 2025 Apr 25;16(1):615. doi: 10.1007/s12672-025-02386-6. Discov Oncol. 2025. PMID: 40281359 Free PMC article. Review.
References
-
- Ruiz‐Ortega M, Rayego‐Mateos S, Lamas S, Ortiz A, Rodrigues‐Diez RR. Targeting the progression of chronic kidney disease. Nat Rev Nephrol. 2020;16(5):269‐288. - PubMed
-
- Tang PM, Nikolic‐Paterson DJ, Lan HY. Macrophages: versatile players in renal inflammation and fibrosis. Nat Rev Nephrol. 2019;15(3):144‐158. - PubMed
-
- Liu BC, Tang TT, Lv LL, Lan HY. Renal tubule injury: a driving force toward chronic kidney disease. Kidney Int. 2018;93(3):568‐579. - PubMed
MeSH terms
Substances
Grants and funding
- 2018ZX09711001-001-017/National Major Science and Technology Projects of China
- 202102130501005/Shanxi Provincial Key Research and Development Project
- 82003770/National Natural Science Foundation of China
- 201901D211545/Natural Science Foundation of Shanxi Province
- 202403021211185/Natural Science Foundation of Shanxi Province
LinkOut - more resources
Full Text Sources

