Patient Reported Outcome Measures in cancer care: a hybrid effectiveness-Implementation trial to optimise Symptom control and health service Experience (PROMISE)-protocol for a randomised controlled trial of electronic self-reporting of symptoms versus usual care during and following treatment in patients with cancer
- PMID: 39566946
- PMCID: PMC11580250
- DOI: 10.1136/bmjopen-2024-090836
Patient Reported Outcome Measures in cancer care: a hybrid effectiveness-Implementation trial to optimise Symptom control and health service Experience (PROMISE)-protocol for a randomised controlled trial of electronic self-reporting of symptoms versus usual care during and following treatment in patients with cancer
Abstract
Introduction: Routine collection of patient-reported outcome measures (PROMs) has the potential to inform and improve cancer care. It is now feasible for patients to complete PROMs electronically (ePROMs) providing information about their current levels of symptoms, side effects of treatment and other concerns. PROM scores can be tracked over time allowing more timely identification of problems and more appropriate intervention. Studies have reported clear benefits in patient-clinician communication when PROMs are used and trials in the USA and France found patients randomised to complete regular ePROMs reported better health-related quality of life, had fewer unplanned hospital visits and, importantly, significantly better survival than those randomised to usual care. However, information about the effects on health outcomes and, particularly, the cost-effectiveness of incorporating this information into practice is limited.
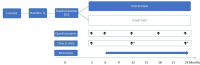
Methods and analysis: PROMISE (Patient Reported Outcome Measures in cancer care: a hybrid effectiveness-Implementation trial to optimise Symptom control and health service Experience) is a multicentre, randomised hybrid effectiveness/implementation trial to evaluate the clinical and cost-effectiveness of using ePROMs in routine cancer care to improve patient outcomes. Participants (target sample=572; randomised 1:1 to intervention and control) are adults aged 18 years or older diagnosed with a solid cancer and starting treatment at one of the four study hospitals. The primary outcomes are unplanned hospital presentations and physical/functional well-being at 6 months. We hypothesise that, compared with usual care, patients randomised to use an ePROM tool will have fewer unplanned hospital presentations, report better health-related quality of life and greater satisfaction with their care and that the ePROM tool will be cost-effective. We will also assess implementation and process outcomes consistent with the RE-AIM (Reach Effectiveness Adoption Implementation Maintenance) Framework.
Ethics and dissemination: This trial has been approved by the Metro South Human Research Ethics Committee (HREC/2020/QMS/67441). Participants provide written informed consent, including consent for record linkage, prior to completing the baseline questionnaire. Study results will be disseminated via peer-reviewed journals and presentations at scientific conferences and clinical meetings.
Trial registration number: ACTRN12620001290987.
Keywords: Adult oncology; Implementation Science; Patient Reported Outcome Measures; Quality of Life; Randomized Controlled Trial.
© Author(s) (or their employer(s)) 2024. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: PMW has received a speaker’s fee from AstraZeneca (December, 2021). ME and DW were part of the Metro North Health team that developed AboutMe and BB and LRW were part of the PAH team that developed My Health My Way but neither tool has been commercialised and none of the authors have any financial interests in the tools. RL has received grant funding from MSD for an unrelated study and sits on Advisory Boards for/has received honoraria and travel funding from MSD, L’Oreal and Sanofi. BB has been a member of the Metro South Human Research Ethics Committee since 2018 but was not involved in the review of this study.
Figures
Similar articles
-
Improving the patient-centred care of children with life-altering skin conditions using feedback from electronic patient-reported outcome measures: protocol for a hybrid effectiveness-implementation study (PEDS-ePROM).BMJ Open. 2021 Apr 9;11(4):e041861. doi: 10.1136/bmjopen-2020-041861. BMJ Open. 2021. PMID: 33837095 Free PMC article.
-
Self-reported MeasUrement of Physical and PsychosOcial Symptoms Response Tool (SUPPORT-dialysis): systematic symptom assessment and management in patients on in-centre haemodialysis - a parallel arm, non-randomised feasibility pilot study protocol.BMJ Open. 2024 Jan 30;14(1):e080712. doi: 10.1136/bmjopen-2023-080712. BMJ Open. 2024. PMID: 38296283 Free PMC article.
-
Implementing physical activity for individuals with cancer during treatment: protocol for the IMPACT implementation-effectiveness trial.BMJ Open. 2025 Mar 27;15(3):e101013. doi: 10.1136/bmjopen-2025-101013. BMJ Open. 2025. PMID: 40148001 Free PMC article.
-
Telephone interventions for symptom management in adults with cancer.Cochrane Database Syst Rev. 2020 Jun 2;6(6):CD007568. doi: 10.1002/14651858.CD007568.pub2. Cochrane Database Syst Rev. 2020. PMID: 32483832 Free PMC article.
-
Assessing Patient-Reported Outcomes in Routine Cancer Clinical Care Using Electronic Administration and Telehealth Technologies: Realist Synthesis of Potential Mechanisms for Improving Health Outcomes.J Med Internet Res. 2023 Nov 28;25:e48483. doi: 10.2196/48483. J Med Internet Res. 2023. PMID: 38015606 Free PMC article. Review.
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical