Brain and behavioural anomalies caused by Tbx1 haploinsufficiency are corrected by vitamin B12
- PMID: 39567195
- PMCID: PMC11579592
- DOI: 10.26508/lsa.202403075
Brain and behavioural anomalies caused by Tbx1 haploinsufficiency are corrected by vitamin B12
Abstract
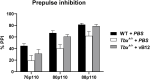
The brain-related phenotypes observed in 22q11.2 deletion syndrome (DS) patients are highly variable, and their origin is poorly understood. Changes in brain metabolism might contribute to these phenotypes, as many of the deleted genes are involved in metabolic processes, but this is unknown. This study shows for the first time that Tbx1 haploinsufficiency causes brain metabolic imbalance. We studied two mouse models of 22q11.2DS using mass spectrometry, nuclear magnetic resonance spectroscopy, and transcriptomics. We found that Tbx1 +/- mice and Df1/+ mice, with a multigenic deletion that includes Tbx1, have elevated brain methylmalonic acid, which is highly brain-toxic. Focusing on Tbx1 mutants, we found that they also have a more general brain metabolomic imbalance that affects key metabolic pathways, such as glutamine-glutamate and fatty acid metabolism. We provide transcriptomic evidence of a genotype-vitamin B12 treatment interaction. In addition, vitamin B12 treatment rescued a behavioural anomaly in Tbx1 +/- mice. Further studies will be required to establish whether the specific metabolites affected by Tbx1 haploinsufficiency are potential biomarkers of brain disease status in 22q11.2DS patients.
© 2024 Caterino et al.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures











References
-
- Paylor R, Glaser B, Mupo A, Ataliotis P, Spencer C, Sobotka A, Sparks C, Choi C-H, Oghalai J, Curran S, et al. (2006) Tbx1 haploinsufficiency is linked to behavioral disorders in mice and humans: Implications for 22q11 deletion syndrome. Proc Natl Acad Sci U S A 103: 7729–7734. 10.1073/pnas.0600206103 - DOI - PMC - PubMed
-
- Torres-Juan L, Rosell J, Morla M, Vidal-Pou C, García-Algas F, de la Fuente M-A, Juan M, Tubau A, Bachiller D, Bernues M, et al. (2007) Mutations in TBX1 genocopy the 22q11.2 deletion and duplication syndromes: A new susceptibility factor for mental retardation. Eur J Hum Genet 15: 658–663. 10.1038/sj.ejhg.5201819 - DOI - PubMed
-
- Ogata T, Niihori T, Tanaka N, Kawai M, Nagashima T, Funayama R, Nakayama K, Nakashima S, Kato F, Fukami M, et al. (2014) TBX1 mutation identified by exome sequencing in a Japanese family with 22q11.2 deletion syndrome-like craniofacial features and hypocalcemia. PLoS One 9: e91598. 10.1371/journal.pone.0091598 - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
