Copper homeostasis and cuproptosis in central nervous system diseases
- PMID: 39567497
- PMCID: PMC11579297
- DOI: 10.1038/s41419-024-07206-3
Copper homeostasis and cuproptosis in central nervous system diseases
Abstract
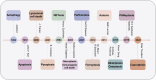
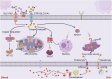
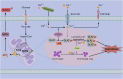
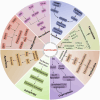
Copper (Cu), an indispensable micronutrient for the sustenance of living organisms, contributes significantly to a vast array of fundamental metabolic processes. The human body maintains a relatively low concentration of copper, which is mostly found in the bones, liver, and brain. Despite its low concentration, Cu plays a crucial role as an indispensable element in the progression and pathogenesis of central nervous system (CNS) diseases. Extensive studies have been conducted in recent years on copper homeostasis and copper-induced cell death in CNS disorders, including glioma, Alzheimer's disease, Amyotrophic lateral sclerosis, Huntington's disease, and stroke. Cuproptosis, a novel copper-induced cell death pathway distinct from apoptosis, necrosis, pyroptosis, and ferroptosis, has been identified as potentially intricately linked to the pathogenic mechanisms underlying various CNS diseases. Therefore, a systematic review of copper homeostasis and cuproptosis and their relationship with CNS disorders could deepen our understanding of the pathogenesis of these diseases. In addition, it may provide new insights and strategies for the treatment of CNS disorders.
© 2024. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures

Similar articles
-
Cope with copper: From molecular mechanisms of cuproptosis to copper-related kidney diseases.Int Immunopharmacol. 2024 May 30;133:112075. doi: 10.1016/j.intimp.2024.112075. Epub 2024 Apr 24. Int Immunopharmacol. 2024. PMID: 38663316 Review.
-
Copper Metabolism and Cuproptosis: Molecular Mechanisms and Therapeutic Perspectives in Neurodegenerative Diseases.Curr Med Sci. 2024 Feb;44(1):28-50. doi: 10.1007/s11596-024-2832-z. Epub 2024 Feb 10. Curr Med Sci. 2024. PMID: 38336987 Review.
-
Cuproptosis and physical training.Clin Hemorheol Microcirc. 2024;88(3):337-350. doi: 10.3233/CH-242329. Clin Hemorheol Microcirc. 2024. PMID: 39031346 Review.
-
Copper homeostasis and cuproptosis in health and disease.MedComm (2020). 2024 Sep 17;5(10):e724. doi: 10.1002/mco2.724. eCollection 2024 Oct. MedComm (2020). 2024. PMID: 39290254 Free PMC article. Review.
-
Roles and mechanisms of copper homeostasis and cuproptosis in osteoarticular diseases.Biomed Pharmacother. 2024 May;174:116570. doi: 10.1016/j.biopha.2024.116570. Epub 2024 Apr 9. Biomed Pharmacother. 2024. PMID: 38599063 Review.
Cited by
-
Polystyrene nanoplastics exposure induces cognitive impairment in mice via induction of oxidative stress and ERK/MAPK-mediated neuronal cuproptosis.Part Fibre Toxicol. 2025 May 20;22(1):13. doi: 10.1186/s12989-025-00633-w. Part Fibre Toxicol. 2025. PMID: 40394693 Free PMC article.
-
Copper metabolism in hepatocellular carcinoma: from molecular mechanisms to therapeutic opportunities.Front Mol Biosci. 2025 May 13;12:1578693. doi: 10.3389/fmolb.2025.1578693. eCollection 2025. Front Mol Biosci. 2025. PMID: 40433591 Free PMC article. Review.
-
Decoding Parkinson's Disease: The interplay of cell death pathways, oxidative stress, and therapeutic innovations.Redox Biol. 2025 Jul 23;85:103787. doi: 10.1016/j.redox.2025.103787. Online ahead of print. Redox Biol. 2025. PMID: 40712453 Free PMC article. Review.
-
Inhibition of the cGAS-STING pathway via an endogenous copper ion-responsive covalent organic framework nanozyme for Alzheimer's disease treatment.Chem Sci. 2025 Mar 11;16(17):7215-7226. doi: 10.1039/d4sc07963a. eCollection 2025 Apr 30. Chem Sci. 2025. PMID: 40144496 Free PMC article.
-
Nanobubble-mediated sonodynamic therapy enhances cuproptosis in the treatment of hepatocellular carcinoma.Nanoscale Adv. 2025 Jun 12;7(15):4651-4659. doi: 10.1039/d5na00280j. eCollection 2025 Jul 22. Nanoscale Adv. 2025. PMID: 40556860 Free PMC article.
References
-
- Zeinali T, Salmani F, Naseri K. Dietary intake of cadmium, chromium, copper, nickel, and lead through the consumption of meat, liver, and kidney and assessment of human health risk in Birjand, southeast of Iran. Biol Trace Elem Res. 2019;191:338–47. - PubMed
-
- Mezzaroba L, Alfieri DF, Colado Simão AN, Vissoci Reiche EM. The role of zinc, copper, manganese and iron in neurodegenerative diseases. Neurotoxicology. 2019;74:230–41. - PubMed
-
- Qian Y, Mikeska G, Harris ED, Bratton GR, Tiffany-Castiglioni E. Effect of lead exposure and accumulation on copper homeostasis in cultured C6 rat glioma cells. Toxicol Appl Pharm. 1999;158:41–9. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

