Bladder cancer detection in urine by novel methylation markers
- PMID: 39567591
- PMCID: PMC11579363
- DOI: 10.1038/s41598-024-77781-0
Bladder cancer detection in urine by novel methylation markers
Abstract
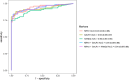
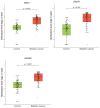
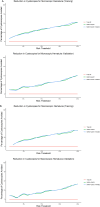
Although cystoscopy is a reliable tool for detecting bladder cancer (BC) in patients with hematuria, it is invasive, costly and often unnecessary since most patients with hematuria do not have BC. Consequently, developing urinary biomarkers for non-invasive BC detection is a major clinical need. While DNA methylation markers hold promise, diagnostic performance can still be improved. We assessed 11 candidate methylation markers for urinary BC detection. Urine samples from 77 primary BC patients and 69 controls were used for marker selection and training, with independent validation conducted on samples from 63 primary BC patients and 71 controls. Samples were self-collected at home, mailed to the hospital and analyzed via quantitative methylation-specific polymerase chain reaction. Marker performance was evaluated through univariable and multivariable logistic regression analyses. Decision curve analysis (DCA) gauged clinical utility by potential cystoscopy reduction. Evaluation identified three most promising markers: NRN1, GALR1, and HAND2. These markers exhibited significantly elevated methylation levels in BC compared to controls in both cohorts (P < 0.001). The combined marker set demonstrated an area under the curve (AUC) of 0.94 at 84% (95% CI: 76-92%) sensitivity and 96% (95% CI: 91-100%) specificity. Validation yielded nearly equivalent accuracy (AUC 0.89, sensitivity 76% (95% CI: 65-86%), specificity 93% (95% CI: 86-99%)). DCA indicated a potential of 20 to 35% reduction in cystoscopies depending on the clinical scenario. The excellent diagnostic potential of our methylation markers for non-invasive BC detection, emphasizes their significance for future diagnostic strategies.
Keywords: Biomarker; Bladder cancer; DNA methylation; Detection; Liquid biopsy; Urinary biomarker; Urine.
© 2024. The Author(s).
Conflict of interest statement
Declarations. Competing interests: Renske D. M. Steenbergen holds a minority stake in Self-screen B.V., a spin-off company of Amsterdam UMC, location VUmc, which owns patents and products related to the development of methylation markers for cervical screening. All other authors do not have any competing interest to declare. Ethics approval and consent to participate: The study was conducted in accordance with the Declaration of Helsinki. The study protocol was approved by the Medical Ethics Committee (2018.355 [16-10-2018], WO 18.155 [21-12-2018]) and all participants provided informed written consent before inclusion in the study.
Figures

References
-
- Yafi, F. A., Brimo, F., Steinberg, J., Aprikian, A. G., Tanguay, S. & Kassouf, W. Prospective analysis of sensitivity and specificity of urinary cytology and other urinary biomarkers for bladder cancer. Urol. Oncol.33(2), 66 e25–31. 10.1016/j.urolonc.2014.06.008 (2015). - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

