A foldon-free prefusion F trimer vaccine for respiratory syncytial virus to reduce off-target immune responses
- PMID: 39567664
- PMCID: PMC11602707
- DOI: 10.1038/s41564-024-01860-1
A foldon-free prefusion F trimer vaccine for respiratory syncytial virus to reduce off-target immune responses
Abstract
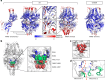
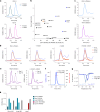
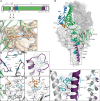
Respiratory syncytial virus (RSV) is a major cause of severe respiratory disease in infants and older people. Current RSV subunit vaccines are based on a fusion protein that is stabilized in the prefusion conformation and linked to a heterologous foldon trimerization domain to obtain a prefusion F (preF) trimer. Here we show that current RSV vaccines induce undesirable anti-foldon antibodies in non-human primates, mice and humans. To overcome this, we designed a foldon-free RSV preF trimer by elucidating the structural basis of trimerization-induced preF destabilization through molecular dynamics simulations and by introducing amino acid substitutions that negate hotspots of charge repulsion. The highly stable prefusion conformation was validated using antigenic and cryo-electron microscopy analysis. The preF is immunogenic and protective in naive mouse models and boosts neutralizing antibody titres in RSV-pre-exposed mice and non-human primates, while achieving similar titres to approved RSV vaccines in mice. This stable preF design is a promising option as a foldon-independent candidate for a next-generation RSV vaccine immunogen.
© 2024. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing financial interests. M.J.G.B., A.K., J.J. and J.P.M.L. are co-inventors on related vaccine patents. M.J.G.B., F.C., X.Y., D.v.O., L.L., W.v.d.H., J. V., A.T., R.V., J.J., L.v.d.F., R.Z. and J.P.M.L. are current or former employees of Johnson and Johnson.
Figures



References
-
- Rha, B. et al. Respiratory syncytial virus-associated hospitalizations among young children: 2015–2016. Pediatrics146, e20193611 (2020). - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

