Cephalopod-inspired jetting devices for gastrointestinal drug delivery
- PMID: 39567682
- PMCID: PMC11634773
- DOI: 10.1038/s41586-024-08202-5
Cephalopod-inspired jetting devices for gastrointestinal drug delivery
Abstract
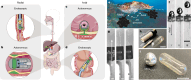
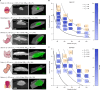
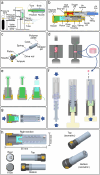
Needle-based injections currently enable the administration of a wide range of biomacromolecule therapies across the body, including the gastrointestinal tract1-3, through recent developments in ingestible robotic devices4-7. However, needles generally require training, sharps management and disposal, and pose challenges for autonomous ingestible systems. Here, inspired by the jetting systems of cephalopods, we have developed and evaluated microjet delivery systems that can deliver jets in axial and radial directions into tissue, making them suitable for tubular and globular segments of the gastrointestinal tract. Furthermore, they are implemented in both tethered and ingestible formats, facilitating endoscopic applications or patient self-dosing. Our study identified suitable pressure and nozzle dimensions for different segments of the gastrointestinal tract and applied microjets in a variety of devices that support delivery across the various anatomic segments of the gastrointestinal tract. We characterized the ability of these systems to administer macromolecules, including insulin, a glucagon-like peptide-1 (GLP1) analogue and a small interfering RNA (siRNA) in large animal models, achieving exposure levels similar to those achieved with subcutaneous delivery. This research provides key insights into jetting design parameters for gastrointestinal administration, substantially broadening the possibilities for future endoscopic and ingestible drug delivery devices.
© 2024. The Author(s).
Conflict of interest statement
Competing interests: D. Sticker, A.G., T.D., B.M., J.P.H.J., D. Schultz, E.M.D.A., M. Poulsen, C.A., H.D.P., N.E.E., C.C., A.H.U., M. Pereverzina, R.K.K., C.M.D., S.B.G., I.P., A.B., A.A., M.R.F., P.H., U.L.R., J.J.W. and S.T.B. are employees and shareholders of Novo Nordisk. G.T. has received consulting fees and grant funding from Novo Nordisk. B.M., J.P.H.J. and M.R.F. are inventors of the following patent: ‘Jensen, B. et al. Medical Device with Actuation Mechanism. WO2020157324 (6 August 2020)’. G.A., D. Sticker, A.G., Y.L., D.G., B.M., J.W., J.P.H.J., T.S.L., C.C., S.Y., A.B., N.R., J.J.W. and G.T. are inventors of the following patent: ‘Arrick, G. et al. Drug Delivery Device. WO2022034041 (17 February 2022)’. G.A., D. Sticker, A.G., D.G., J.W., J.P.H.J., M. Poulsen, C.C., J.J.W. and G.T. are inventors of the following patent: ‘Traverso, G. et al. Ingestible Drug Delivery Device. WO2022035750 (17 February 2022)’. The other authors declare no competing interests.
Figures











References
-
- Smeets, F. G. M. et al. Endoscopic botulinum toxin for gastroparesis: results of a retrospective series. Gastrointest. Disord.1, 164–175 (2019).
-
- Nelson, D. B. et al. ASGE technology status evaluation report. Injection needles. February 1999. Gastrointest. Endosc.50, 928–931 (1999). - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

