Exaptation of ancestral cell-identity networks enables C4 photosynthesis
- PMID: 39567684
- PMCID: PMC11618092
- DOI: 10.1038/s41586-024-08204-3
Exaptation of ancestral cell-identity networks enables C4 photosynthesis
Abstract
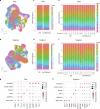
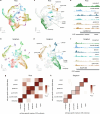
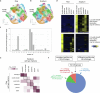
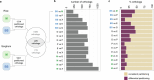
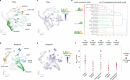
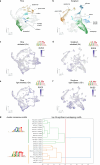
C4 photosynthesis is used by the most productive plants on the planet, and compared with the ancestral C3 pathway, it confers a 50% increase in efficiency1. In more than 60 C4 lineages, CO2 fixation is compartmentalized between tissues, and bundle-sheath cells become photosynthetically activated2. How the bundle sheath acquires this alternate identity that allows efficient photosynthesis is unclear. Here we show that changes to bundle-sheath gene expression in C4 leaves are associated with the gain of a pre-existing cis-code found in the C3 leaf. From single-nucleus gene-expression and chromatin-accessibility atlases, we uncover DNA binding with one finger (DOF) motifs that define bundle-sheath identity in the major crops C3 rice and C4 sorghum. Photosynthesis genes that are rewired to be strongly expressed in the bundle-sheath cells of C4 sorghum acquire cis-elements that are recognized by DOFs. Our findings are consistent with a simple model in which C4 photosynthesis is based on the recruitment of an ancestral cis-code associated with bundle-sheath identity. Gain of such elements harnessed a stable patterning of transcription factors between cell types that are found in both C3 and C4 leaves to activate photosynthesis in the bundle sheath. Our findings provide molecular insights into the evolution of the complex C4 pathway, and might also guide the rational engineering of C4 photosynthesis in C3 crops to improve crop productivity and resilience3,4.
© 2024. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures











References
-
- Ghannoum, O., Evans, J. R. & von Caemmerer, S. in C4 Photosynthesis and Related CO2Concentrating Mechanisms (eds Raghavendra, A. & Sage, R.) 129–146 (Springer, 2011).
-
- Sage, R. F., Christin, P. A. & Edwards, E. J. The C4 plant lineages of planet Earth. J. Exp. Bot.62, 3155–3169 (2011). - PubMed
-
- Hibberd, J. M., Sheehy, J. E. & Langdale, J. A. Using C4 photosynthesis to increase the yield of rice-rationale and feasibility. Curr. Opin. Plant Biol.11, 228–231 (2008). - PubMed
-
- Sage, R. F. The evolution of C4 photosynthesis. New Phytol.161, 341–370 (2004). - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

