Social state alters vision using three circuit mechanisms in Drosophila
- PMID: 39567699
- PMCID: PMC11735400
- DOI: 10.1038/s41586-024-08255-6
Social state alters vision using three circuit mechanisms in Drosophila
Abstract
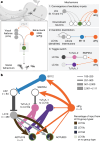
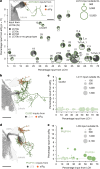
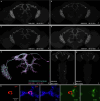
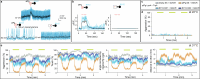
Animals are often bombarded with visual information and must prioritize specific visual features based on their current needs. The neuronal circuits that detect and relay visual features have been well studied1-8. Much less is known about how an animal adjusts its visual attention as its goals or environmental conditions change. During social behaviours, flies need to focus on nearby flies9-11. Here we study how the flow of visual information is altered when female Drosophila enter an aggressive state. From the connectome, we identify three state-dependent circuit motifs poised to modify the response of an aggressive female to fly-sized visual objects: convergence of excitatory inputs from neurons conveying select visual features and internal state; dendritic disinhibition of select visual feature detectors; and a switch that toggles between two visual feature detectors. Using cell-type-specific genetic tools, together with behavioural and neurophysiological analyses, we show that each of these circuit motifs is used during female aggression. We reveal that features of this same switch operate in male Drosophila during courtship pursuit, suggesting that disparate social behaviours may share circuit mechanisms. Our study provides a compelling example of using the connectome to infer circuit mechanisms that underlie dynamic processing of sensory signals.
© 2024. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures










Update of
-
Social state gates vision using three circuit mechanisms in Drosophila.bioRxiv [Preprint]. 2024 Mar 17:2024.03.15.585289. doi: 10.1101/2024.03.15.585289. bioRxiv. 2024. Update in: Nature. 2025 Jan;637(8046):646-653. doi: 10.1038/s41586-024-08255-6. PMID: 38559111 Free PMC article. Updated. Preprint.
References
-
- Goodale, M. A. & Milner, A. D. Separate visual pathways for perception and action. Trends Neurosci.15, 20–25 (1992). - PubMed
-
- Shinomiya, K., Nern, A., Meinertzhagen, I. A., Plaza, S. M. & Reiser, M. B. Neuronal circuits integrating visual motion information in Drosophila melanogaster. Curr. Biol.32, 3529–3544 (2022). - PubMed
-
- Perge, J. A., Borghuis, B. G., Bours, R. J. E., Lankheet, M. J. M. & van Wezel, R. J. A. Temporal dynamics of direction tuning in motion-sensitive macaque area MT. J. Neurophysiol.93, 2104–2116 (2005). - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

