Avian raptors are indicator species and victims of high pathogenicity avian influenza virus HPAIV H5N1 (clade 2.3.4.4b) in Germany
- PMID: 39567708
- PMCID: PMC11579473
- DOI: 10.1038/s41598-024-79930-x
Avian raptors are indicator species and victims of high pathogenicity avian influenza virus HPAIV H5N1 (clade 2.3.4.4b) in Germany
Abstract
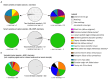
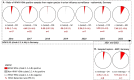
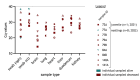
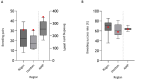
Transition of highly pathogenic clade 2.3.4.4b H5 avian influenza virus (HPAIV) from epizootic to enzootic status in Northern European countries was associated with severe losses and even mass mortalities among various wild bird species. Both avian and mammalian raptors hunting infected debilitated birds or scavenging on virus-contaminated avian carcasses contracted HPAIV infection. This precarious pathogen-prey-predator relation further worsened when in 2021 and 2022 outbreaks in Germany overlapped with the hatching season of avian raptor species. Retro- and prospective surveillance revealed avian raptors as important indicators of HPAIV and its genetic diversity on the one hand. On the other hand, their role as victims of HPAIV is stipulated. The first case of an HPAIV H5N1-related death of a white-tailed sea eagle (Haliaeetus albicilla; WTSE) hatch in Germany, 2021, followed by several such cases in 2022, and a low overall seropositivity rate of 5.0-7.9% among WTSE nestlings, raised fears of a serious negative impact on reproduction rates of WTSEs and other birds of prey when HPAIV becomes enzootic in an ecosystem. However, comparably stable breeding success of WTSE in the study area in 2022 and a potentially evolving natural immunity raises hope for a less severe long-term impact.
Keywords: Haliaeetus albicilla; Highly pathogenic avian influenza virus H5N1; Maternal immunity; Nestling; White-tailed sea eagle; Wild bird surveillance.
© 2024. The Author(s).
Conflict of interest statement
Declarations. Competing interests: The authors declare no competing interests.
Figures


References
-
- Yoon, S. W., Webby, R. J. & Webster, R. G. Evolution and ecology of influenza A viruses. Curr. Top. Microbiol. Immunol.385, 359–375. 10.1007/82_2014_396 (2014). - PubMed
-
- Globig, A. et al. Epidemiological and ornithological aspects of outbreaks of highly pathogenic avian influenza virus H5N1 of Asian lineage in wild birds in Germany, 2006 and 2007. Transbound. Emerg. Dis.56, 57–72. 10.1111/j.1865-1682.2008.01061.x (2009). - PubMed
-
- Globig, A. et al. Consecutive natural influenza A virus infections in sentinel mallards in the evident absence of subtype-specific hemagglutination inhibiting antibodies. Transbound. Emerg. Dis.60, 395–402. 10.1111/j.1865-1682.2012.01357.x (2013). - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

