Molecular condensation of the CO/NF-YB/NF-YC/FT complex gates floral transition in Arabidopsis
- PMID: 39567828
- PMCID: PMC11696179
- DOI: 10.1038/s44318-024-00293-0
Molecular condensation of the CO/NF-YB/NF-YC/FT complex gates floral transition in Arabidopsis
Abstract
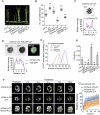
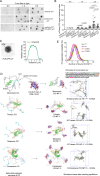
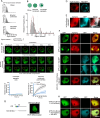
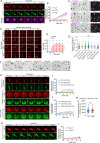
The plant master photoperiodic regulator CONSTANS (CO) interacts with Nuclear Factor-Y subunits B2 (NF-YB2) and C9 (NF-YC9) and transcriptionally activates the florigen gene FLOWERING LOCUS T (FT), regulating floral transition. However, the molecular mechanism of the functional four-component complex assembly in the nucleus remains elusive. We report that co-phase separation of CO with NF-YB2/NF-YC9/FT precisely controls heterogeneous CO assembly and FT transcriptional activation. In response to light signals, CO proteins form functional percolation clusters from a diffuse distribution in a B-box-motif-dependent manner. Multivalent coassembly with NF-YC9 and NF-YB2 prevents inhibitory condensate formation and is necessary to maintain proper CO assembly and material properties. The intrinsically disordered region (IDR) of NF-YC9, containing a polyglutamine motif, fine-tunes the functional properties of CO/NF-YB/NF-YC condensates. Specific FT promoter recognition with polyelectrolyte partitioning also enables the fluidic functional properties of CO/NF-YB/NF-YC/FT condensates. Our findings offer novel insights into the tunable macromolecular condensation of the CO/NF-YB/NF-YC/FT complex in controlling flowering in the photoperiod control.
Keywords: CO/NF-Y; Floral Transition; Macromolecular Condensation; Photoperiodic Regulation.
© 2024. The Author(s).
Conflict of interest statement
Disclosure and competing interests statement. The authors declare no competing interests.
Figures




Similar articles
-
Structural insights into the multivalent binding of the Arabidopsis FLOWERING LOCUS T promoter by the CO-NF-Y master transcription factor complex.Plant Cell. 2021 May 31;33(4):1182-1195. doi: 10.1093/plcell/koab016. Plant Cell. 2021. PMID: 33693873
-
CONSTANS Imparts DNA Sequence Specificity to the Histone Fold NF-YB/NF-YC Dimer.Plant Cell. 2017 Jun;29(6):1516-1532. doi: 10.1105/tpc.16.00864. Epub 2017 May 19. Plant Cell. 2017. PMID: 28526714 Free PMC article.
-
The Nuclear Factor Y subunits NF-YB2 and NF-YB3 play additive roles in the promotion of flowering by inductive long-day photoperiods in Arabidopsis.Planta. 2008 Oct;228(5):709-23. doi: 10.1007/s00425-008-0773-6. Epub 2008 Jul 4. Planta. 2008. PMID: 18600346
-
The control of flowering in time and space.J Exp Bot. 2006;57(13):3415-8. doi: 10.1093/jxb/erl159. Epub 2006 Sep 27. J Exp Bot. 2006. PMID: 17005922 Review.
-
Circadian clock and photoperiodic response in Arabidopsis: from seasonal flowering to redox homeostasis.Biochemistry. 2015 Jan 20;54(2):157-70. doi: 10.1021/bi500922q. Epub 2014 Dec 30. Biochemistry. 2015. PMID: 25346271 Free PMC article. Review.
Cited by
-
Phase Separation: Orchestrating Biological Adaptations to Environmental Fluctuations.Int J Mol Sci. 2025 May 12;26(10):4614. doi: 10.3390/ijms26104614. Int J Mol Sci. 2025. PMID: 40429758 Free PMC article. Review.
-
Salinity Stress Induces Phase Separation of Plant BARENTSZ to Form Condensates.Rice (N Y). 2025 Aug 11;18(1):75. doi: 10.1186/s12284-025-00830-3. Rice (N Y). 2025. PMID: 40788520 Free PMC article.
-
Phase separation as a key mechanism in plant development, environmental adaptation, and abiotic stress response.J Biol Chem. 2025 Jun;301(6):108548. doi: 10.1016/j.jbc.2025.108548. Epub 2025 Apr 24. J Biol Chem. 2025. PMID: 40286852 Free PMC article. Review.
References
-
- An H, Roussot C, Suárez-López P, Corbesier L, Vincent C, Piñeiro M, Hepworth S, Mouradov A, Justin S, Turnbull C et al (2004) CONSTANS acts in the phloem to regulate a systemic signal that induces photoperiodic flowering of Arabidopsis. Development 131:3615–3626 - PubMed
-
- Andrés F, Coupland G (2012) The genetic basis of flowering responses to seasonal cues. Nat Rev Genet 13:627–639 - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

