Melanin-like nanoparticles slow cyst growth in ADPKD by dual inhibition of oxidative stress and CREB
- PMID: 39567834
- PMCID: PMC11730739
- DOI: 10.1038/s44321-024-00167-2
Melanin-like nanoparticles slow cyst growth in ADPKD by dual inhibition of oxidative stress and CREB
Abstract
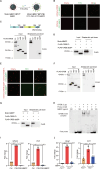
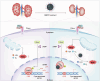
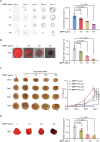
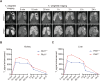
Melanin-like nanoparticles (MNPs) have recently emerged as valuable agents in antioxidant therapy due to their excellent biocompatibility and potent capacity to scavenge various reactive oxygen species (ROS). However, previous studies have mainly focused on acute ROS-related diseases, leaving a knowledge gap regarding their potential in chronic conditions. Furthermore, apart from their well-established antioxidant effects, it remains unclear whether MNPs target other intracellular molecular pathways. In this study, we synthesized ultra-small polyethylene glycol-incorporated Mn2+-chelated MNP (MMPP). We found that MMPP traversed the glomerular filtration barrier and specifically accumulated in renal tubules. Autosomal dominant polycystic kidney disease (ADPKD) is a chronic genetic disorder closely associated with increased oxidative stress and featured by the progressive enlargement of cysts originating from various segments of the renal tubules. Treatment with MMPP markedly attenuated oxidative stress levels, inhibited cyst growth, thereby improving renal function. Interestingly, we found that MMPP effectively inhibits a cyst-promoting gene program downstream of the cAMP-CREB pathway, a crucial signaling pathway implicated in ADPKD progression. Mechanistically, we observed that MMPP directly binds to the bZIP DNA-binding domain of CREB, leading to competitive inhibition of CREB's DNA binding ability and subsequent reduction in CREB target gene expression. In summary, our findings identify an intracellular target of MMPP and demonstrate its potential for treating ADPKD by simultaneously targeting oxidative stress and CREB transcriptional activity.
Keywords: ADPKD Therapy; Melanin-like Nanoparticle; Reactive Oxygen Species; cAMP-CREB Pathway.
© 2024. The Author(s).
Conflict of interest statement
Disclosure and competing interests statement. The authors declare no competing interests.
Figures
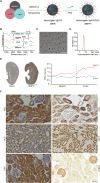

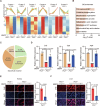
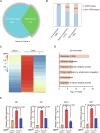
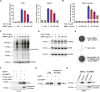
Similar articles
-
Integrative Cistromic and Transcriptomic Analyses Identify CREB Target Genes in Cystic Renal Epithelial Cells.J Am Soc Nephrol. 2021 Oct;32(10):2529-2541. doi: 10.1681/ASN.2021010101. Epub 2021 Jun 23. J Am Soc Nephrol. 2021. PMID: 34162733 Free PMC article.
-
Cardamonin retards progression of autosomal dominant polycystic kidney disease via inhibiting renal cyst growth and interstitial fibrosis.Pharmacol Res. 2020 May;155:104751. doi: 10.1016/j.phrs.2020.104751. Epub 2020 Mar 6. Pharmacol Res. 2020. PMID: 32151678
-
HL156A, an AMP-Activated Protein Kinase Activator, Inhibits Cyst Growth in Autosomal Dominant Polycystic Kidney Disease.Biomolecules. 2024 Jul 7;14(7):806. doi: 10.3390/biom14070806. Biomolecules. 2024. PMID: 39062520 Free PMC article.
-
Cell Proliferation and Apoptosis in ADPKD.Adv Exp Med Biol. 2016;933:25-34. doi: 10.1007/978-981-10-2041-4_3. Adv Exp Med Biol. 2016. PMID: 27730432 Review.
-
Multidrug therapy for polycystic kidney disease: a review and perspective.Am J Nephrol. 2013;37(2):175-82. doi: 10.1159/000346812. Epub 2013 Feb 15. Am J Nephrol. 2013. PMID: 23428809 Review.
Cited by
-
Melanin-like nanoparticles as a potential novel therapeutic approach in ADPKD.EMBO Mol Med. 2025 Jan;17(1):6-8. doi: 10.1038/s44321-024-00173-4. Epub 2024 Nov 20. EMBO Mol Med. 2025. PMID: 39567833 Free PMC article.
References
-
- Augustine R, Hasan A, Primavera R, Wilson RJ, Thakor AS, Kevadiya BD (2020) Cellular uptake and retention of nanoparticles: Insights on particle properties and interaction with cellular components. Mater Today Commun 25:101692
-
- Bao X, Zhao J, Sun J, Hu M, Yang X (2018) Polydopamine nanoparticles as efficient scavengers for reactive oxygen species in periodontal disease. ACS Nano 12:8882–8892 - PubMed
MeSH terms
Substances
Grants and funding
- 32070634/MOST | National Natural Science Foundation of China (NSFC)
- 82071982/MOST | National Natural Science Foundation of China (NSFC)
- 92068113/MOST | National Natural Science Foundation of China (NSFC)
- 32241018/MOST | National Natural Science Foundation of China (NSFC)
- 82070689/MOST | National Natural Science Foundation of China (NSFC)
- 82200832/MOST | National Natural Science Foundation of China (NSFC)
- 82200834/MOST | National Natural Science Foundation of China (NSFC)
- 21JCJQJC00100/| Natural Science Foundation of Tianjin Municipality ()
- 19JCJQJC63700/| Natural Science Foundation of Tianjin Municipality ()
- 19JCJQJC63800/| Natural Science Foundation of Tianjin Municipality ()
- 2022ZD054/Tianjin Municipal Education Commission ()
- 2021M692407/China Postdoctoral Science Foundation (China Postdoctoral Foundation Project)
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

