Rotavirus rewires host cell metabolic pathways toward glutamine catabolism for effective virus infection
- PMID: 39567865
- PMCID: PMC11583611
- DOI: 10.1080/19490976.2024.2428425
Rotavirus rewires host cell metabolic pathways toward glutamine catabolism for effective virus infection
Abstract
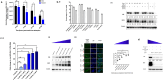
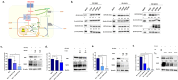
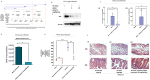
Rotavirus (RV) accounts for 19.11% of global diarrheal deaths. Though GAVI assisted vaccine introduction has curtailed RV induced mortality, factors like RV strain diversity, differential infantile gut microbiome, malnutrition, interference from maternal antibodies and other administered vaccines, etc. often compromise vaccine efficacy. Herein emerges the need of antivirals which can be administered adjunct to vaccination to curb the socio-economic burden stemming from frequent RV infection. Cognisance of pathogen-perturbed host cellular physiology has revolutionized translational research and aided precision-based therapy, particularly for viruses, with no metabolic machinery of their own. To date there has been limited exploration of the host cellular metabolome in context of RV infection. In this study, we explored the endometabolomic landscape of human intestinal epithelial cells (HT-29) on RV-SA11 infection. Significant alteration of host cellular metabolic pathways like the nucleotide biosynthesis pathway, alanine, aspartate and glutamate metabolism pathway, the host citric acid cycle was observed in RV-SA11 infection scenario. Detailed study further revealed that RV replication is exclusively dependent on glutamine metabolism for their propagation in host cells. Glutamine metabolism generates glutamate, aspartate, and asparagine which facilitates virus infection. Abrogation of aspartate biogenesis from glutamine by use of Aminooxyacetic acid (AOAA), significantly curbed RV-SA11 infection in-vitro and in-vivo. Overall, the study improves our understanding of host-rotavirus interactome and recognizes host glutamine metabolism pathway as a suitable target for effective therapeutic intervention against RV infection.
Keywords: Rotavirus; antiviral; aspartate aminotransferase; glutamine metabolism; metabolomics.
Conflict of interest statement
No potential conflict of interest was reported by the author(s).
Figures


Similar articles
-
Rotavirus NSP1 Contributes to Intestinal Viral Replication, Pathogenesis, and Transmission.mBio. 2021 Dec 21;12(6):e0320821. doi: 10.1128/mBio.03208-21. Epub 2021 Dec 14. mBio. 2021. PMID: 34903043 Free PMC article.
-
Rotavirus Degrades Multiple Interferon (IFN) Type Receptors To Inhibit IFN Signaling and Protects against Mortality from Endotoxin in Suckling Mice.J Virol. 2017 Dec 14;92(1):e01394-17. doi: 10.1128/JVI.01394-17. Print 2018 Jan 1. J Virol. 2017. PMID: 29070687 Free PMC article.
-
Rotavirus Reprograms Multiple Interferon Receptors and Restricts Their Intestinal Antiviral and Inflammatory Functions.J Virol. 2020 Feb 28;94(6):e01775-19. doi: 10.1128/JVI.01775-19. Print 2020 Feb 28. J Virol. 2020. PMID: 31896593 Free PMC article.
-
Rotavirus Interactions With Host Intestinal Epithelial Cells.Front Immunol. 2021 Dec 22;12:793841. doi: 10.3389/fimmu.2021.793841. eCollection 2021. Front Immunol. 2021. PMID: 35003114 Free PMC article. Review.
-
The Complex Interactions Between Rotavirus and the Gut Microbiota.Front Cell Infect Microbiol. 2021 Jan 8;10:586751. doi: 10.3389/fcimb.2020.586751. eCollection 2020. Front Cell Infect Microbiol. 2021. PMID: 33489932 Free PMC article. Review.
Cited by
-
Tapping into Metabolomics for Understanding Host and Rotavirus Group A Interactome.Life (Basel). 2025 May 10;15(5):765. doi: 10.3390/life15050765. Life (Basel). 2025. PMID: 40430193 Free PMC article.
References
-
- Whitaker-Dowling P, Youngner JS.. Virus-host cell interactions. Encycl Of Virol. 1999;1957:1961. DOI: 10.1006/rwvi.1999.0343 - DOI
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
