Common gastrointestinal symptoms in healthy infants receiving goat milk-based formula or cow's milk-based formula: a double-blind, randomized, controlled trial
- PMID: 39567910
- PMCID: PMC11577781
- DOI: 10.1186/s12887-024-05214-y
Common gastrointestinal symptoms in healthy infants receiving goat milk-based formula or cow's milk-based formula: a double-blind, randomized, controlled trial
Abstract
Objective: To assess common gastrointestinal symptoms in healthy Brazilian infants receiving goat milk-based formula (GMF) compared to cow's milk-based formula (CMF).
Methods: We performed a 24-weeks double-blind, randomized, controlled study in Brazil, enrolling healthy infants from 3 to 12 months of age. Primary outcome were the gastrointestinal (GI) symptoms stool consistency, regurgitation frequency and crying duration. Secondary outcomes were growth trajectories and hemoglobin levels. Repeated mixed models were used to compare outcomes variables between GMF and CMF groups, while adjusting for age at baseline.
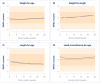
Results: Fifty-six infants were recruited and randomly allocated in the GMF (n = 26) and the CMF (n = 30) group. Scores on all measured GI symptoms were low and similar among the groups throughout intervention period and improved over time. Average age- and sex-adjusted WHO z-scores of weight, length, head circumference, and weight-for-length were all within +/-1 SD and similar between groups, indicating adequate growth. Serum hemoglobin was 11.1 (SD 0.7) g/dL in infants fed GMF and 11.0 (SD 0.8) g/dL in infants fed CMF after the intervention and was similar between groups.
Conclusion: GMF was well tolerated, safe and supported adequate growth in infants. This was shown by the low occurrence of GI symptoms, adequate blood hemoglobin levels and adequate growth within WHO standards.
Trial registration: The clinical trial was approved by the ethics committee of the Federal University of Bahia under number CAAE06923319.5.0000.5577. The study was retrospectively registered in clinicaltrials.gov on 02/05/2024 under identifier NCT06395571.
Keywords: Breast-milk substitutes; Goat milk-based infant formula; Infant Nutrition; Infant formula.
© 2024. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: The clinical trial was approved by the ethics committee of the Federal University of Bahia under number CAAE 06923319.5.0000.5577 and the trial was conducted according to the guidelines laid down in the Declaration of Helsinki. Written informed consent was obtained from the parent(s) or legal guardian(s) prior to start of the trial. Consent for publication: Not applicable. Competing interests: The authors LL, YMK and LZ are employed at Ausnutria B.V., which manufactured the goat milk formula used in the study. PM and HCRJ declare no conflict of interest.
Figures
Similar articles
-
Randomized, double-blind comparison of growth in infants receiving goat milk formula versus cow milk infant formula.J Paediatr Child Health. 2005 Nov;41(11):564-8. doi: 10.1111/j.1440-1754.2005.00722.x. J Paediatr Child Health. 2005. PMID: 16398838 Clinical Trial.
-
Whole Goat Milk-Based Formula versus Whey-Based Cow Milk Formula: What Formula Do Infants Enjoy More?-A Feasibility, Double-Blind, Randomized Controlled Trial.Nutrients. 2023 Sep 19;15(18):4057. doi: 10.3390/nu15184057. Nutrients. 2023. PMID: 37764840 Free PMC article. Clinical Trial.
-
Sleep duration among breastfed, goat milk-based or cow's milk-based infant formula-fed infants: Post hoc analyses from a double-blind RCT.J Pediatr Gastroenterol Nutr. 2025 Mar;80(3):482-489. doi: 10.1002/jpn3.12436. Epub 2024 Dec 19. J Pediatr Gastroenterol Nutr. 2025. PMID: 39698907 Free PMC article. Clinical Trial.
-
Infant formulas containing hydrolysed protein for prevention of allergic disease.Cochrane Database Syst Rev. 2018 Oct 19;10(10):CD003664. doi: 10.1002/14651858.CD003664.pub6. Cochrane Database Syst Rev. 2018. PMID: 30338526 Free PMC article.
-
Dietary modifications for infantile colic.Cochrane Database Syst Rev. 2018 Oct 10;10(10):CD011029. doi: 10.1002/14651858.CD011029.pub2. Cochrane Database Syst Rev. 2018. PMID: 30306546 Free PMC article.
References
-
- WHO. Exclusive breastfeeding for six months best for babies everywhere. 2011 [cited 2023 August 15]; https://www.who.int/news/item/15-01-2011-exclusive-breastfeeding-for-six...
-
- Darmon N, Drewnowski A. Does social class predict diet quality? Am J Clin Nutr. 2008;87(5):1107–17. - PubMed
-
- Keefe DM. Agency Response Letter GRAS Notice No. GRN 000644, C.f.F.S.a.A. Nutrition, Editor. 2016.
-
- (EFSA). E.F.S.A., scientific opinion on the suitability of goat milk protein as a source of protein in infant formula and in follow-on formula. EFSA J. 2012;10(3):2603.
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical