Towards Optimal Automated 68Ga-Radiolabeling Conditions of the DOTA-Bisphosphonate BPAMD Without Pre-Purification of the Generator Eluate
- PMID: 39568296
- PMCID: PMC11641010
- DOI: 10.1002/jlcr.4128
Towards Optimal Automated 68Ga-Radiolabeling Conditions of the DOTA-Bisphosphonate BPAMD Without Pre-Purification of the Generator Eluate
Abstract
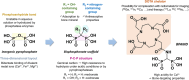
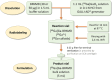
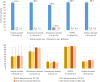
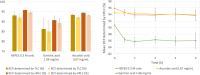
DOTA-functionalized bisphosphonates can be useful tools for PET imaging of bone metastases when radiolabeled with 68Ga. Moreover, the versatility of DOTA allows the complexation of radiometals with therapeutic applications (e.g., 177Lu), positioning these bisphosphonates as attractive theranostic agents. Among these molecules, BPAMD is a compound whose radiolabeling with 68Ga has already been described, but only through manual methods. Thus, a fully automated protocol for 68Ga radiolabeling of BPAMD on the GAIA® ± LUNA® synthesis module was designed, and a thorough study of the radiolabeling conditions was undertaken. [68Ga]Ga-BPAMD was produced in good radiochemical purity (> 93%) and high radiochemical yield (> 91%) using 0.3 M HEPES buffer. The nature of the reaction vessel showed no significant effect on the radiolabeling outcome. Similarly, addition of an antiradiolysis compound to the reaction medium did not significantly improve the already excellent stability of [68Ga]Ga-BPAMD over time. The radiolabeled product obtained by automated synthesis was evaluated in vivo in healthy mice and confirmed high accumulation in the joints and along the backbone.
© 2024 The Author(s). Journal of Labelled Compounds and Radiopharmaceuticals published by John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures





Similar articles
-
"One Method to Label Them All": A Single Fully Automated Protocol for GMP-Compliant 68Ga Radiolabeling of PSMA-11, Transposable to PSMA-I&T and PSMA-617.Curr Radiopharm. 2024;17(3):285-301. doi: 10.2174/0118744710293461240219111852. Curr Radiopharm. 2024. PMID: 38424422 Free PMC article.
-
(68)Ga-BPAMD: PET-imaging of bone metastases with a generator based positron emitter.Nucl Med Biol. 2012 Oct;39(7):993-9. doi: 10.1016/j.nucmedbio.2012.04.007. Epub 2012 May 23. Nucl Med Biol. 2012. PMID: 22633217
-
Simplified NaCl based (68)Ga concentration and labeling procedure for rapid synthesis of (68)Ga radiopharmaceuticals in high radiochemical purity.Bioconjug Chem. 2012 Aug 15;23(8):1712-7. doi: 10.1021/bc300103t. Epub 2012 Jul 19. Bioconjug Chem. 2012. PMID: 22755505
-
Automation synthesis modules review.Appl Radiat Isot. 2013 Jun;76:38-45. doi: 10.1016/j.apradiso.2012.09.010. Epub 2012 Sep 26. Appl Radiat Isot. 2013. PMID: 23084477 Review.
-
A Review of Accelerator-Produced Ga-68 with Solid Targets.Curr Radiopharm. 2021;14(4):315-324. doi: 10.2174/1874471013666201224113651. Curr Radiopharm. 2021. PMID: 33357189 Review.
Cited by
-
Specific reaction conditions for efficient automated 68Ga-radiolabeling of the FAP-2286 pseudopeptide on a GAIA® synthesizer.Front Med (Lausanne). 2025 Jul 22;12:1628158. doi: 10.3389/fmed.2025.1628158. eCollection 2025. Front Med (Lausanne). 2025. PMID: 40766056 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

