Acute myeloid leukemia drug-tolerant persister cells survive chemotherapy by transiently increasing plasma membrane rigidity, that also increases their sensitivity to immune cell killing
- PMID: 39568440
- PMCID: PMC11962361
- DOI: 10.3324/haematol.2024.286018
Acute myeloid leukemia drug-tolerant persister cells survive chemotherapy by transiently increasing plasma membrane rigidity, that also increases their sensitivity to immune cell killing
Abstract
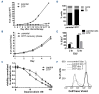
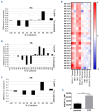
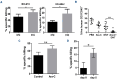
Resistance to chemotherapy remains a major hurdle to the cure of patients with acute myeloid leukemia (AML). Recent studies indicate that a minority of malignant cells, termed drug-tolerant persisters (DTP), stochastically upregulate stress pathways to evade cell death upon acute exposure to chemotherapy without acquiring new genetic mutations. This chemoresistant state is transient and the cells return to the baseline state after removal of chemotherapy. Nevertheless, the mechanisms employed by DTP to resist chemotherapy are not well understood and it is largely unknown whether these mechanisms are also seen in patients receiving chemotherapy. Here, we used leukemia cell lines, primary AML patients' samples and samples from patients with AML receiving systemic chemotherapy to study the DTP state. We demonstrated that a subset of AML cells transiently increases membrane rigidity to resist killing due to acute exposure to daunorubicin and Ara-C. Upon removal of the chemotherapy, membrane rigidity returned to baseline and the cells regained chemosensitivity. Although resistant to chemotherapy, the increased membrane rigidity rendered AML cells more susceptible to T-cell-mediated killing. Thus, we identified a novel mechanism by which DTP leukemic cells evade chemotherapy and a strategy to eradicate these persistent cells.
Figures


Similar articles
-
Drug-tolerant persister cells in acute myeloid leukemia: pressing challenge and promising new strategies for treatment.Front Med (Lausanne). 2025 May 14;12:1586552. doi: 10.3389/fmed.2025.1586552. eCollection 2025. Front Med (Lausanne). 2025. PMID: 40443513 Free PMC article. Review.
-
Human stem cell factor-antibody [anti-SCF] enhances chemotherapy cytotoxicity in human CD34+ resistant myeloid leukaemia cells.Leuk Res. 2006 Mar;30(3):296-302. doi: 10.1016/j.leukres.2005.06.026. Epub 2005 Aug 19. Leuk Res. 2006. PMID: 16112192
-
Radotinib enhances cytarabine (Ara-C)-induced acute myeloid leukemia cell death.BMC Cancer. 2020 Dec 4;20(1):1193. doi: 10.1186/s12885-020-07701-8. BMC Cancer. 2020. PMID: 33276759 Free PMC article.
-
ERK Activity in Immature Leukemic Cells Drives Clonal Selection during Induction Therapy for Acute Myeloid Leukemia.Sci Rep. 2020 May 20;10(1):8349. doi: 10.1038/s41598-020-65061-6. Sci Rep. 2020. PMID: 32433559 Free PMC article.
-
Daunorubicin and Cytarabine Liposome in Newly Diagnosed Therapy-Related Acute Myeloid Leukemia (AML) or AML With Myelodysplasia-Related Changes.Ann Pharmacother. 2018 Aug;52(8):792-800. doi: 10.1177/1060028018764923. Epub 2018 Mar 13. Ann Pharmacother. 2018. PMID: 29532662 Review.
Cited by
-
Drug-tolerant persister cells in acute myeloid leukemia: pressing challenge and promising new strategies for treatment.Front Med (Lausanne). 2025 May 14;12:1586552. doi: 10.3389/fmed.2025.1586552. eCollection 2025. Front Med (Lausanne). 2025. PMID: 40443513 Free PMC article. Review.
References
-
- Rücker FG, Schlenk RF, Bullinger L, et al. . TP53 alterations in acute myeloid leukemia with complex karyotype correlate with specific copy number alterations, monosomal karyotype, and dismal outcome. Blood. 2012;119(9):2114-2121. - PubMed
-
- Zeng AGX, Bansal S, Jin L, et al. . A cellular hierarchy framework for understanding heterogeneity and predicting drug response in acute myeloid leukemia. Nat Med. 2022;28(6):1212-1223. - PubMed
-
- Ofran Y, Hayun M, Leiba R, et al. . Bone marrow blast elimination by the fifth day of 7 + 3 induction is the strongest predictor of potential cure in patients with acute myeloid leukemia younger than 61 years of age: a long-term follow-up of a multi-center prospective study. Am J Hematol. 2020;95(1):E3-E5. - PubMed
-
- Bertoli S, Bories P, Béné MC, et al. . Prognostic impact of day 15 blast clearance in risk-adapted remission induction chemotherapy for younger patients with acute myeloid leukemia: long-term results of the multicenter prospective LAM-2001 trial by the GOELAMS study group. Haematologica. 2014;99(1):46-53. - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

